��Ŀ����
��ij�¶��£���100mL Cu��IO3��2��Һ�м��������ľ��ữ��KI��Һ���������µķ�Ӧ����KI+Cu��IO3��2+H2SO4--I2+H2O+K2SO4+CuSO4��CuSO4+KI--CuI��+I2+K2SO4��ַ�Ӧ����0.10mol/L��Na2S2O3��Һ�ζ���I2+2Na2S2O3 2NaI+Na2S4O6������������52.00mL��Na2S2O3��Һ��������������⣺��1������ƽ���еĢ١�������Ӧ��
��2����Ҫ����ԭCu��IO3��2��Һ�����ʵ���Ũ�ȣ�����������������
��������1�����ݢ�KI+Cu��IO3��2+H2SO4--I2+H2O+K2SO4+CuSO4��CuSO4+KI--CuI��+I2+K2SO4����ʽ�������л��ϼ�KI�� I��-1�۱�ΪI2��0�۵�I��Cu��IO3��2�� I��+5�۱�ΪI2��0�ۣ����л��ϼ�CuSO4��Cu��+2�۱�ΪCuI��Cu�۵�+I��KI�� I��+1�۱�ΪI2��0�ۣ������������õ��ĵ��ӣ����ڻ�ԭ��ʧȥ�ĵ�����⣻
��2�����ݢ٢���ƽ�õķ���ʽ���ɵù�ϵʽ��2Cu��IO3��2��13I2��26 Na2S2O3����⣮
��2�����ݢ٢���ƽ�õķ���ʽ���ɵù�ϵʽ��2Cu��IO3��2��13I2��26 Na2S2O3����⣮
����⣺��ƽ������ԭ����ʽ��ͨ����������С���������䷢��������ԭ������ǰ��ϵ����������ԭ���غ���δδ����������ԭ������ǰ��ϵ������С���������������裺1����üۣ��귴Ӧ����������л��ϼ۱仯��Ԫ�أ� 2���б仯�� �г���Ӧǰ��Ԫ�صĻ��ϼ۱仯��3����������ʹ���ϼ����ߺͽ��͵�������ȣ�4����ϵ��������ԭ���غ���ƽ�������ʵ�ϵ����
��1����KI+Cu��IO3��2+H2SO4--I2+H2O+K2SO4+CuSO4
���ϼ�-1 0+5 0
��ԭ��K I-------------I2 1��Iʧ1�����ӣ������� Cu��IO3��2--------------I2 2��I��10�����ӣ�
������С������������ԭ��K Iǰϵ��Ϊ10��������Cu��IO3��2ǰϵ��Ϊ1������I �غ㣬I2ǰϵ��Ϊ6��������ԭ���غ���ƽ�������ʵ�ϵ�����ʢٴ�Ϊ��
��10KI+Cu��IO3��2+6H2SO4=6I2+6H2O+5K2SO4+CuSO4
��CuSO4+KI--CuI��+I2+K2SO4
���ϼ�-1 0+2+1
��ԭ��K I-------------I2 1��Iʧ1�����ӣ�������CuSO4-------------CuI��1��Cu��1�����ӣ�
������С������������ԭ��K Iǰϵ��Ϊ1��������CuSO4ǰϵ��Ϊ1������I �غ㣬I2ǰϵ��Ϊ1/2��������ԭ���غ���ƽ�������ʵ�ϵ�������ʽ����ͬ����2��
�ʢڴ�Ϊ��2CuSO4+4KI=2CuI��+I2+2K2SO4
��2��n��Na2S2O3��=c��Na2S2O3����v��Na2S2O3��=0.10mol/L��52.00mL=5.2����10-3 mol
I2 +2Na2S2O3=2NaI+Na2S4O6
1 2
n��I2�� 5.2��10-3 mol
n��I2��=2.6��10-3 mol
����
��10KI+Cu��IO3��2+6H2SO4=6I2+6H2O+5K2SO4+CuSO4
��2CuSO4+4KI=2CuI��+I2+2K2SO4
���١�2+�ڵã�
20KI+2Cu��IO3��2+12H2SO4+2CuSO4+4KI=12I2+12H2O+10K2SO4+2CuSO4+2CuI��+I2+2K2SO4
������ȥ��ͬ��2CuSO4�ϲ���ͬ��ã�
4KI+2Cu��IO3��2+12H2SO4=13I2+12H2O+12K2SO4+2CuI��
2 13
n[Cu��IO3��2]n��I2��=2.6��10-3 mol
n[Cu��IO3��2]=4��10-4mol��
c[Cu��IO3��2]=nCu��IO3��2]��v[Cu��IO3��2]=4��10-3 mol/L
�ʴ�Ϊ��ԭCu��IO3��2��Һ�����ʵ���Ũ��c[Cu��IO3��2]=4��10-3mol/L
��1����KI+Cu��IO3��2+H2SO4--I2+H2O+K2SO4+CuSO4
���ϼ�-1 0+5 0
��ԭ��K I-------------I2 1��Iʧ1�����ӣ������� Cu��IO3��2--------------I2 2��I��10�����ӣ�
������С������������ԭ��K Iǰϵ��Ϊ10��������Cu��IO3��2ǰϵ��Ϊ1������I �غ㣬I2ǰϵ��Ϊ6��������ԭ���غ���ƽ�������ʵ�ϵ�����ʢٴ�Ϊ��
��10KI+Cu��IO3��2+6H2SO4=6I2+6H2O+5K2SO4+CuSO4
��CuSO4+KI--CuI��+I2+K2SO4
���ϼ�-1 0+2+1
��ԭ��K I-------------I2 1��Iʧ1�����ӣ�������CuSO4-------------CuI��1��Cu��1�����ӣ�
������С������������ԭ��K Iǰϵ��Ϊ1��������CuSO4ǰϵ��Ϊ1������I �غ㣬I2ǰϵ��Ϊ1/2��������ԭ���غ���ƽ�������ʵ�ϵ�������ʽ����ͬ����2��
�ʢڴ�Ϊ��2CuSO4+4KI=2CuI��+I2+2K2SO4
��2��n��Na2S2O3��=c��Na2S2O3����v��Na2S2O3��=0.10mol/L��52.00mL=5.2����10-3 mol
I2 +2Na2S2O3=2NaI+Na2S4O6
1 2
n��I2�� 5.2��10-3 mol
n��I2��=2.6��10-3 mol
����
��10KI+Cu��IO3��2+6H2SO4=6I2+6H2O+5K2SO4+CuSO4
��2CuSO4+4KI=2CuI��+I2+2K2SO4
���١�2+�ڵã�
20KI+2Cu��IO3��2+12H2SO4+2CuSO4+4KI=12I2+12H2O+10K2SO4+2CuSO4+2CuI��+I2+2K2SO4
������ȥ��ͬ��2CuSO4�ϲ���ͬ��ã�
4KI+2Cu��IO3��2+12H2SO4=13I2+12H2O+12K2SO4+2CuI��
2 13
n[Cu��IO3��2]n��I2��=2.6��10-3 mol
n[Cu��IO3��2]=4��10-4mol��
c[Cu��IO3��2]=nCu��IO3��2]��v[Cu��IO3��2]=4��10-3 mol/L
�ʴ�Ϊ��ԭCu��IO3��2��Һ�����ʵ���Ũ��c[Cu��IO3��2]=4��10-3mol/L
����������������ԭ��ƽ���õķ�������С���������������ö������ʽ���ҳ���ϵʽ���⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��
��2012?��������ģ���о��Ϳ���CO2��CO�Ĵ��������ǻ�����������Դ���õ�˫Ӯ���⣮CO�����ںϳɼ״�����ѹǿΪ0.1MPa�����£������Ϊb L���ܱ������г���a mol CO��2a mol H2���ڴ��������ºϳɼ״���CO��g��+2H2��g��?CH3OH��g����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��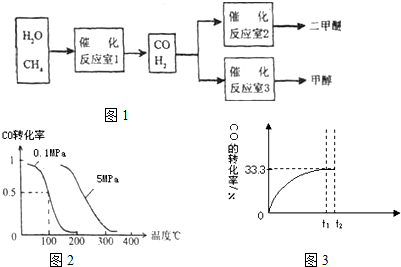
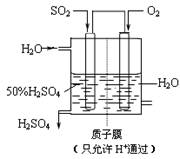
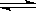 SO3��g�� ��H����98
kJ��mol��1��
SO3��g�� ��H����98
kJ��mol��1��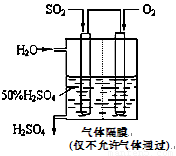
 SO3��g�� ��H����98 kJ��mol��1��
SO3��g�� ��H����98 kJ��mol��1��