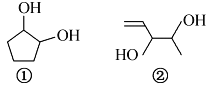��Ŀ����
����Ŀ�����������Ӷ�̨�������г�������ܺϸ��ʽϵͣ����ڽϴ�İ�ȫ�����������ܻ���������һ����Ҫ���棬�ܻ�������࣬�����ڱ��������������Ҫ��һ���࣬![]() ������DMP����һ�ֳ��õ��������������������������ܶ�Ϊ97����ҵ������DMP��������ͼ��ʾ��
������DMP����һ�ֳ��õ��������������������������ܶ�Ϊ97����ҵ������DMP��������ͼ��ʾ��

��֪��R-X+NaOH![]() R-OH+NaX
R-OH+NaX
��1��������Ӧ�ݵķ�Ӧ����Ϊ___________________��
��2��D�й����ŵ�����Ϊ___________________��
��3��DMP�Ľṹ��ʽΪ___________________��
��4��B��C�Ļ�ѧ����ʽΪ_____________________________��
���𰸡� ȡ����Ӧ��������Ӧ�� �Ȼ� 
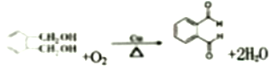
���������ڶ��ױ���������һ�������·���ȡ����Ӧ�����ȴ���A��A���������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ�B��B������������������ȩC��C������������������D��D�ʹ�����������ȡ������Ӧ����DMP������DMP�Ľṹ֪�ڶ��ױ��м���1����ԭ�ӱ���ԭ��ȡ��������A�Ľṹ��ʽΪ��![]() ��B��
��B��![]() ��C��
��C��![]() ��D��
��D��![]() ���ڶ�������ʹ�����������Ӧ����DMP��DMP����������������ܶ�Ϊ97����DMP����Է�������Ϊ194����ô��Ǽ״�����DMP�Ľṹ��ʽΪ��
���ڶ�������ʹ�����������Ӧ����DMP��DMP����������������ܶ�Ϊ97����DMP����Է�������Ϊ194����ô��Ǽ״�����DMP�Ľṹ��ʽΪ��![]() ���ݴ˴�����
���ݴ˴�����
��1����������ķ�����֪������ת����������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��������Ӧ����
![]() �й����ŵ�����Ϊ�Ȼ���
�й����ŵ�����Ϊ�Ȼ���
DMP�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
B��C�ķ�Ӧ�Ǵ��Ĵ���������Ӧ����ѧ����ʽΪ![]() .
.

����Ŀ������������һ����Ҫ�Ĺ�ҵ���Σ�ijͬѧ��������������������ʵ�飺(��֪:Na2O2+2NO=2NaNO2��Na2O2+2NO2=2NaNO3)

��1����ͬѧ�����������Ʊ�NaNO2����װ�õ�����˳��ΪA��___��___��___��___��E��_________(����ţ����ظ�)
��2������a������Ϊ______________��
��3��NO��E�пɱ�������NO3-��д����Ӧ�����ӷ���ʽ_________________________��
��4����ɫ���ⶨ��Ʒ�е�NaNO2����:
����5���б�ŵĴ��̶��Թ��зֱ���벻ͬ����NaNO2��Һ��������1mL��M��Һ(M��NaNO2���Ϻ�ɫ��NaNO2��Ũ��Խ����ɫԽ��)���ټ�����ˮ���������Ϊ10 mL�����Ƴɱ�ɫ��:
�Թܱ�� | a | b | c | d | e |
NaNO2����/(mg.L-1) | 0 | 20 | 40 | 60 | 80 |
�ڳ���0.10g�Ƶõ���Ʒ������ˮ���500mL��Һ��ȡ5mL ����Һ������1mLM��Һ���ټ�����ˮ��10mL ���������ɫ�ױȽϣ�
�۱�ɫ�Ľ����: ����Һ����ɫ��d ���ɫ����ͬ������Ʒ��NaNO2����������Ϊ_______��
��5���ζ����ⶨ��Ʒ�е�NaNO2����:
�ٳ���0.5000g�Ƶõ���Ʒ������ˮ���500mL��Һ��ȡ25.00mL����Һ����ƿ�У�����s mL KI ������Һ(����)��������Ӧ2NO2-+2I-+4H+=2NO��+I2+2H2O��
�ڵ���2~3 ��_____��ָʾ������0.0100mol/LNa2S2O3��Һ���еζ���������______����ʱ����Ϊʹ���յ�(��֪��2 Na2S2O3+ I2=Na2S4O6+2NaI)��
���ظ�ʵ���ƽ������Na2S2O3��Һ�����Ϊ20.50mL������Ʒ��NaNO2����������Ϊ____(����3 λ��Ч����)��
�����в����ᵼ�²ⶨ���ƫ�ߵ���______(�����)��
A.�ζ�����������ƿ�м�����ˮ
B.�ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
C.�۲����ʱ���ζ�ǰ���ӣ��ζ�����
D.�ζ�ʱҡƿ���ȹ������Һ�ε�ƿ��