��Ŀ����
(10��)ͨ�����ǰѲ�1mol ij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С�����ڹ��㻯ѧ��Ӧ�ķ�Ӧ�ȣ���H����
��1����Ҫ��������
a 2HCl(g) �� H2(g)��Cl2(g)����H=
b N2 (g)+3H2(g)= 2NH3(g) ��H="-92" kJ/mol����N��H���ļ����� kJ��mol
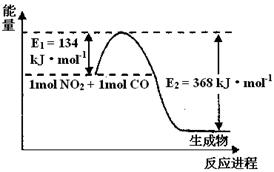
��2��1 mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ
��3�� ��֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H1
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H2
�� H2O(g) �� H2O(l) ��H3
��CH3OH(l)��O2(g)=" CO(g)" + 2H2O(l)��H = ���ú���H1����H2����H3��ʽ�ӱ�ʾ��
(4)��֪��2Al (s)+ 3/2O2��g��==Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
2Fe (s) +3/2O2��g��==Fe2O3(s) ��H=" -815.88" kJ? mol-1
��д����������������ĩ�������ȷ�Ӧ���Ȼ�ѧ����ʽ_______________________��
| ��ѧ�� | Cl��Cl | H��H | H��Cl | N��N |
| ����/kJ��mol | 243 | 436 | 431 | 946 |
a 2HCl(g) �� H2(g)��Cl2(g)����H=
b N2 (g)+3H2(g)= 2NH3(g) ��H="-92" kJ/mol����N��H���ļ����� kJ��mol
��2��1 mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ

��3�� ��֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H1
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H2
�� H2O(g) �� H2O(l) ��H3
��CH3OH(l)��O2(g)=" CO(g)" + 2H2O(l)��H = ���ú���H1����H2����H3��ʽ�ӱ�ʾ��
(4)��֪��2Al (s)+ 3/2O2��g��==Al2O3(s) ��H=" -1" 644.3 kJ? mol-1
2Fe (s) +3/2O2��g��==Fe2O3(s) ��H=" -815.88" kJ? mol-1
��д����������������ĩ�������ȷ�Ӧ���Ȼ�ѧ����ʽ_______________________��
��1����H ="+183" kJ/mol ��2�֣� 391��2�֣�
��2��NO2(g) + CO(g) = CO2(g) + NO(g) ��H =" -" 234kJ��mol-1��2�֣�
��3��1/2��H1-1/2/��H2+2��H3��2�֣�
��4��2Al (s) + Fe2O3(s) === Al2O3(s) +2Fe (s) ��H=" -828.42" kJ mol-1��2�֣�
��2��NO2(g) + CO(g) = CO2(g) + NO(g) ��H =" -" 234kJ��mol-1��2�֣�
��3��1/2��H1-1/2/��H2+2��H3��2�֣�
��4��2Al (s) + Fe2O3(s) === Al2O3(s) +2Fe (s) ��H=" -828.42" kJ mol-1��2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 O2��g��====H2O��l����H����285kJ��mol��1
O2��g��====H2O��l����H����285kJ��mol��1 ����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1
����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1 O2(g)��H2O(l) ����H 3����285.8kJ/mol
O2(g)��H2O(l) ����H 3����285.8kJ/mol  ��
�� aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2
aʱ��lmol H2��ȫȼ������Һ̬ˮ���ų�2 85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� ��
85��8kJ��������1 mol CH4��ȫȼ������Һ̬ˮ��CO2���壬�ų�890��3kJ�������������Ȼ�ѧ����ʽ��д��ȷ���� �� �� ��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol
��+2 O2��g��=CO2��g��+2H2O��1������H=-890��3kJ��mol
 4
4