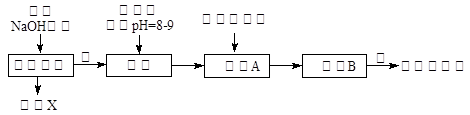��Ŀ����
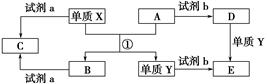
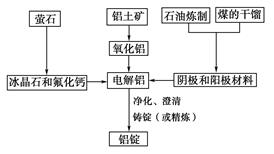
�������㷺�����л��ϳɡ�ӡȾ��ҵ�ȡ���ҵ��������Ϊԭ�ϣ���Ҫ�ɷ�ΪAl��������Al2O3��Fe2O3��SiO2��CaO��MgO�ȣ��Ʊ��������Ĺ����������£�
��֪��Al��OH��3�������ܽ��pH���±���
�ش��������⣺
��1������ʱ����������Ӧ�����ӷ���ʽΪ________________________________��
��2�����������е�һ�μ��������pH��7.0��Ŀ����__________________________________________________________��
pH��7.0ʱ����Һ��c��Al3������________��ͨ�������£�Ksp[Al��OH��3]��1.3��10��33����
��3�����������ʸ��ᡢ��ķ�Ӧ�����ڼ��ܡ���pH��7.0�����ܹ����У����������ļ������ʵ���֮��n1��NaOH����n2��HNO3����n3��HNO3����________��
��4������1 t����������������[Al��NO3��3��9H2O]�����������7.5 t���������壬��������������Ԫ�ص���ʧ��Ϊ10%��������������Ԫ�ص�����������

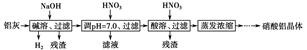
��֪��Al��OH��3�������ܽ��pH���±���
| Al��OH��3 | ��ʼ���� | ������ȫ | ������ʼ�ܽ� | �����ܽ���ȫ |
| pH | 3.3 | 5.0 | 7.8 | 12.8 |
�ش��������⣺
��1������ʱ����������Ӧ�����ӷ���ʽΪ________________________________��
��2�����������е�һ�μ��������pH��7.0��Ŀ����__________________________________________________________��
pH��7.0ʱ����Һ��c��Al3������________��ͨ�������£�Ksp[Al��OH��3]��1.3��10��33����
��3�����������ʸ��ᡢ��ķ�Ӧ�����ڼ��ܡ���pH��7.0�����ܹ����У����������ļ������ʵ���֮��n1��NaOH����n2��HNO3����n3��HNO3����________��
��4������1 t����������������[Al��NO3��3��9H2O]�����������7.5 t���������壬��������������Ԫ�ص���ʧ��Ϊ10%��������������Ԫ�ص�����������
��1��2Al��2OH����2H2O=2AlO2-��3H2��
��2��ʹAl��OH��3������ȫ��1.3��10��12 mol��L��1
��3��1��1��3
��4��60%
��2��ʹAl��OH��3������ȫ��1.3��10��12 mol��L��1
��3��1��1��3
��4��60%
��1��ע�����ҵ���Ҫ�ɷ�ΪAl����Ҫ��Ӧ�������ķ�Ӧ����2���������ͼ��֪������pH��7.0ʱ�����˵õ��ij����ټ�HNO3��������������֪����ΪAl��OH��3��c��Al3������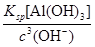 ��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3��
��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3��
��4������Ԫ�ص���������Ϊx
��Al����������������Al��NO3��3��9H2O
��27��������������������375
1 t����1��10%����x��������7.5 t
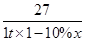 ��
��
��ã�x��0.6����60%��
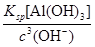 ��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3��
��1.3��10��12 mol��L��1����3�����ܡ���pH��7.0�����ܵķ�Ӧ�ֱ�Ϊ��2Al��2OH����2H2O=2AlO2-��3H2����AlO2-��H����H2O=Al��OH��3����Al��OH��3��3H��=Al3����3H2O����n1��NaOH����n2��HNO3����n3��HNO3����1��1��3����4������Ԫ�ص���������Ϊx
��Al����������������Al��NO3��3��9H2O
��27��������������������375
1 t����1��10%����x��������7.5 t
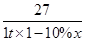 ��
��
��ã�x��0.6����60%��

��ϰ��ϵ�д�
 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
�����Ŀ