��Ŀ����
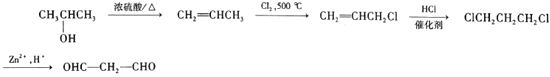
����Ŀ��������֬�ϳɼ������졢ԭ���á�ͿĤ���ۺ��������ã���Ϳ���úϳ���֬�����������;����Ʒ��֮һ�����ͻ���������֬�ĺϳ���·��ͼ��ʾ��

��1��E�Ļ�ѧ������______, D�й����ŵ�������____________��
��2����Ӧ�ٵ��л���Ӧ������_________��
��3������˵����ȷ����_________������ĸ��ţ���
a��������A�ĺ˴Ź���������3���
b����֬�����ǡ���ѿ������ˮ�ⶼ�ܵõ�2������
c. ����B������������ʱ�����Լ���˳�������ǹ�������������Һ����������Һ
d. 1molC ��������������Һ��Ӧ������4 mol Ag
e. 1molE ����ͯ�Ľ����Ʒ�Ӧ����33.6L H2 (��״���£�
��4��д��C![]() D�Ļ�ѧ����ʽ��_________��
D�Ļ�ѧ����ʽ��_________��
��5����Cu ��������F(C8H10O2)��O2����C,��F��ͬ���칹���У��������������ķ����廯����Ľṹ��ʽ��___________��
a����FeCl3��Һ������ɫ��Ӧ��
b��������ȥ��Ӧ�����ɵĻ�����˴Ź���������5��壻
��6����֪��RCH3CH=CH2![]() RCHClCH=CH2�������ϳ�·�ߣ���2-����Ϊԭ�ϣ����Լ���ѡ��������Ʊ�OHC-CH2-CHO�ĺϳ�·��___________��
RCHClCH=CH2�������ϳ�·�ߣ���2-����Ϊԭ�ϣ����Լ���ѡ��������Ʊ�OHC-CH2-CHO�ĺϳ�·��___________��
���𰸡� ������ �Ȼ� ȡ����Ӧ ade ![]()
![]() ��
��![]()
�����������������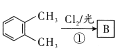 ��Bڱ����������
��BΪ±����������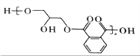 ���ƿ�֪D��
���ƿ�֪D��![]() ��E��CH2OHCHOHCH2OH��
��E��CH2OHCHOHCH2OH��
�������������Ϸ�������1��CH2OHCHOHCH2OH�Ļ�ѧ�����DZ�������![]() �й����ŵ��������Ȼ���
�й����ŵ��������Ȼ���
��2��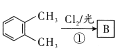 �������ϵ�ȡ����Ӧ��
�������ϵ�ȡ����Ӧ��
��3��a���ڶ��ױ��ṹ�Գƣ��˴Ź���������3�������a��ȷ��
b����ѿ������ˮ��ֻ�õ�������һ����������b������
c. ����B������������ʱ�����Լ���˳�������ǹ�������������Һ�������ᡢ�����������Һ����c������
d. ![]() ����2��ȩ����1mol
����2��ȩ����1mol![]() ��������������Һ��Ӧ������4 mol Ag����d��ȷ��
��������������Һ��Ӧ������4 mol Ag����d��ȷ��
e. CH2OHCHOHCH2OH����3���ǻ���1molE ����ͯ�Ľ����Ʒ�Ӧ����33.6L H2 (��״���£�����e��ȷ��
��4���ڱ�����ȩ��������ͭ����Ϊ�ڱ�������Ļ�ѧ����ʽ��![]() ��
��
��5����Cu ��������F(C8H10O2)��O2����![]() ����F���ڱ����״���Fͬ���칹���У���FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ���������ȥ��Ӧ�����ɵĻ��� ��˴Ź���������5���ķ����廯����Ľṹ��ʽ��
����F���ڱ����״���Fͬ���칹���У���FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ���������ȥ��Ӧ�����ɵĻ��� ��˴Ź���������5���ķ����廯����Ľṹ��ʽ��![]() ��
��![]() ��
��
(6����֪������RCH3CH=CH2![]() RCHClCH=CH2����2-�����Ʊ�OHC-CH2-CHO��Ҫ�Ʊ���ϩ��������������ȡ����Ӧ�������Ȼ��ⷢ���ӳɷ�Ӧ���ϳ�·��Ϊ
RCHClCH=CH2����2-�����Ʊ�OHC-CH2-CHO��Ҫ�Ʊ���ϩ��������������ȡ����Ӧ�������Ȼ��ⷢ���ӳɷ�Ӧ���ϳ�·��Ϊ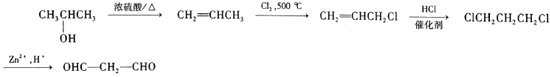

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����3��2 L���ܱ������У�����ͬ���¶��¡�ʹ����ͬ�Ĵ����ֱ���з�Ӧ��
3H2(g)+N2(g) ![]() 2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ�й��������±�������������ȷ����
2NH3(g)������ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ�й��������±�������������ȷ����
������� | ��ʼ��Ӧ�� | �ﵽƽ���ʱ��(min) | ƽ��ʱN2��Ũ��(mol/L) | ƽ��ʱ�����ܶ� |
�� | 3molH2��2molN2 | t1 | c1 | ��1 |
�� | 6molH2��4molN2 | 5 | 1.5 | ��2 |
�� | 2molNH3 | 8 | c3 | ��3 |
A. 2��1=��2>��3
B. �������з�Ӧ�ӿ�ʼ����ƽ��ķ�Ӧ����Ϊv��H2��=0.05mol/(L��min)
C. c1��c3
D. 2c1��1.5


