��Ŀ����
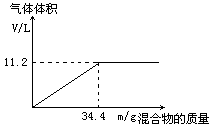
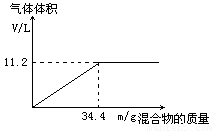
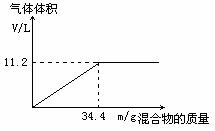
��100mLNaOH��Һ�м���NH4NO3��(NH4)2SO4�Ĺ���������ȳ�ַ�Ӧ����ͼ��ʾ����Ļ����������Ͳ�����������(��״��)��ϵ��
(1)�Լ���NaOH��Һ�����ʵ���Ũ�ȡ�
(2)��NaOH��Һ�����Ϊ140mL���������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?
(3)��NaOH��Һ�����Ϊ180mL�����������������Ϊ51��6g����ַ�Ӧ��������������(��״��)Ϊ������?
�⣺��1��5mol/L ��3�֣� ��2��15.68L��3�֣� ��3��16.8L��3�֣�
����:��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ