��Ŀ����
��2013?���϶�ģ�����Na2CO3�������������о��й㷺����;��ͼ1��ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��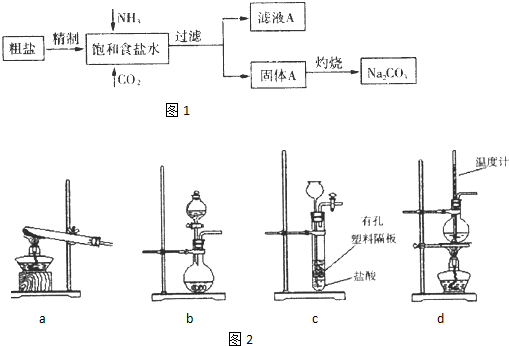
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ͷ�ӦΪNaCl+NH3+CO2+H2O��NaHCO3��+NH4Cl����ش��������⣺
��1�������к��е�����������Ca2+��Mg2+��SO42-�ȣ�
���Ƴ��ӵIJ���˳��a��
a�������ܽ⣬��ȥ������b�����������pH��c������Ba��OH��2��Һ��d������Na2CO3��Һ��e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2��������
��2�����չ���A��Na2CO3��
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4+�ķ�����
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH=13��Na+��K+����Һ�м�������NH4HCO3��ʹpH���ͣ���Ӧ�����ӷ���ʽ
��3��ͼ2װ���г�����ʵ�����Ʊ�CO2����
��4��һ����Ȼ���ɷ���aNa2CO3?bNa2CO3?cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ����������������ѡ�����ʵ�鷽����ȫ����ѡ����Լ���1mol?L-1H2SO4��Һ��1.0mol?L-1BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca��OH��2��Һ������ˮ��
�ٳ�ȡm1gһ������Ȼ�����Ʒ��������������ˮ�У�
��
��
�ܼ�����Ȼ����к�Na2CO3������������

��֪����ʳ��ˮ��ͨ��NH3��CO2�����ͷ�ӦΪNaCl+NH3+CO2+H2O��NaHCO3��+NH4Cl����ش��������⣺
��1�������к��е�����������Ca2+��Mg2+��SO42-�ȣ�
���Ƴ��ӵIJ���˳��a��
c
c
��d
d
��e
e
��b������ĸ��ţ���a�������ܽ⣬��ȥ������b�����������pH��c������Ba��OH��2��Һ��d������Na2CO3��Һ��e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2��������
NH3������ˮ�������������ܽ�Ȳ����CO2
NH3������ˮ�������������ܽ�Ȳ����CO2
����2�����չ���A��Na2CO3��
a
a
������ĸ��ţ��н��У�a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4+�ķ�����
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+
������ҺA�����ؽᾧ�ܹ����NH4HCO3����pH=13��Na+��K+����Һ�м�������NH4HCO3��ʹpH���ͣ���Ӧ�����ӷ���ʽ
NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O
NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O
����3��ͼ2װ���г�����ʵ�����Ʊ�CO2����
bc
bc
������ĸ��ţ�����bʾ���װ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ�Ũ��ˮ
Ũ��ˮ
�����Լ����ƣ�����ƿ�ڿɼ���Ĺ����Լ���ʯ�ң���NaOH���壩
��ʯ�ң���NaOH���壩
�����Լ����ƣ�����4��һ����Ȼ���ɷ���aNa2CO3?bNa2CO3?cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ����������������ѡ�����ʵ�鷽����ȫ����ѡ����Լ���1mol?L-1H2SO4��Һ��1.0mol?L-1BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca��OH��2��Һ������ˮ��
�ٳ�ȡm1gһ������Ȼ�����Ʒ��������������ˮ�У�
��
��������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ
��������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ
����
���ˡ�ϴ�ӡ������������
���ˡ�ϴ�ӡ������������
���ܼ�����Ȼ����к�Na2CO3������������
��������1���ݸ���SO42-��Ca 2+��Mg2+����ת��Ϊ����������ȥ���Լ����ݲ��ܲ��������ʵ�Ҫ������ǰ�����Ĺ�����ҺӦ�ú�������Һ��ȥ����������������Һ��NH3������ˮ�������������ܽ�Ȳ����CO2��
��2�����ݹ������������ȷֽ⣻
����NH4+�ļ��鷽����ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��
����NH4+��HCO3-�������������Ʒ�Ӧ��
��3��ʵ������ȡ������̼�ķ�Ӧԭ����������
������Ũ��ˮ�мӼ����ʯ�ң���Ϊ��ˮ�д�������ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-��������ʯ�Ҳ�������������ƽ�����ƣ�ͬʱ�ų��������ȴٽ���ˮ�Ļӷ����Ƶð�����
��4������ʵ���ԭ������̼���ȫ��ת���ɶ�����̼�����ö�����̼�ͳ���ʯ��ˮ����������ͨ���������������������̼�����ʵ�����Na2CO3�����ʵ������������������Na2CO3������������
��2�����ݹ������������ȷֽ⣻
����NH4+�ļ��鷽����ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��
����NH4+��HCO3-�������������Ʒ�Ӧ��
��3��ʵ������ȡ������̼�ķ�Ӧԭ����������
������Ũ��ˮ�мӼ����ʯ�ң���Ϊ��ˮ�д�������ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-��������ʯ�Ҳ�������������ƽ�����ƣ�ͬʱ�ų��������ȴٽ���ˮ�Ļӷ����Ƶð�����
��4������ʵ���ԭ������̼���ȫ��ת���ɶ�����̼�����ö�����̼�ͳ���ʯ��ˮ����������ͨ���������������������̼�����ʵ�����Na2CO3�����ʵ������������������Na2CO3������������
����⣺��1��SO42-��Ca2+��Mg2+�ȷֱ���Ba��OH��2��Һ��Na2CO3��Һ��Ba��OH��2��Һ��Ӧ���ɳ���������ͨ�����˳�ȥ��Na2CO3��Һ�ܳ�ȥ������Ba��OH��2��Һ�������ܳ�ȥ������Na2CO3��Һ��NaOH��Һ������Ӧ�ȼ�Ba��OH��2��Һ�ټ�Na2CO3��Һ�����ˣ����������ᣬNH3������ˮ�������������ܽ�Ȳ����CO2��������ʳ��ˮ����ͨ��NH3����ͨ��CO2��
�ʴ�Ϊ��c��d��e��NH3������ˮ�������������ܽ�Ȳ����CO2��
��2�����չ���̼��������Na2CO3�����������ȷֽ⣬
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��
NH4+��HCO3-�������������Ʒ�Ӧ��NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O��
�ʴ�Ϊ��a��ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O��
��3��ʵ������ȡ������̼��ѡ��ʯ��ʯ��ϡ��������ʯ��ϡ���ᷴӦ��ȡ�����ù���+Һ��
���壻
Ũ��ˮ�мӼ����ʯ�ң���Ϊ��ˮ�д�������ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�������ƽ�����ƣ�ͬʱ�ų��������ȴٽ���ˮ�Ļӷ����Ƶð�����
�ʴ�Ϊ��b c��Ũ��ˮ����ʯ�ң���NaOH���壩��
��4��ʵ���ԭ������̼���ȫ��ת���ɶ�����̼�����ö�����̼�ͳ���ʯ��ˮ����������ͨ���������������������̼�����ʵ�����Na2CO3�����ʵ������������������Na2CO3���������������Խ���Ȼ�����Ʒ��������������ˮ�У���������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ��Ȼ����ˡ�ϴ�ӡ���������������ɣ�
�ʴ�Ϊ����������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ��������������
�ʴ�Ϊ��c��d��e��NH3������ˮ�������������ܽ�Ȳ����CO2��
��2�����չ���̼��������Na2CO3�����������ȷֽ⣬
ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��
NH4+��HCO3-�������������Ʒ�Ӧ��NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O��
�ʴ�Ϊ��a��ȡ������ҺA���Թ��У���������NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤��A�к���NH4+��NH4++HCO3-+2OH-=NH3?H2O+CO32-+H2O��
��3��ʵ������ȡ������̼��ѡ��ʯ��ʯ��ϡ��������ʯ��ϡ���ᷴӦ��ȡ�����ù���+Һ��
| ������ |
Ũ��ˮ�мӼ����ʯ�ң���Ϊ��ˮ�д�������ƽ�⣺NH3+H2O?NH3?H2O?NH4++OH-�������ƽ�����ƣ�ͬʱ�ų��������ȴٽ���ˮ�Ļӷ����Ƶð�����
�ʴ�Ϊ��b c��Ũ��ˮ����ʯ�ң���NaOH���壩��
��4��ʵ���ԭ������̼���ȫ��ת���ɶ�����̼�����ö�����̼�ͳ���ʯ��ˮ����������ͨ���������������������̼�����ʵ�����Na2CO3�����ʵ������������������Na2CO3���������������Խ���Ȼ�����Ʒ��������������ˮ�У���������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ��Ȼ����ˡ�ϴ�ӡ���������������ɣ�
�ʴ�Ϊ����������ϡ���Ტ�ȣ�����������ͨ�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ��������������
�������������Ƽ�ԭ����ȡNa2CO3���漰�����ʵij��Ӻ��ᴿ��������ʵ����������ʵ��Ʊ��ȣ��Ѷ��еȣ�������ѧ������֪ʶ��������

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ