��Ŀ����
��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�á���ش��������⣺
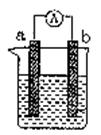
��1�����缫aΪAl���缫bΪCu���������ҺΪϡ����ʱ�������ĵ缫��ӦʽΪ��
��2�����缫aΪAl���缫bΪMg���������ҺΪ����������Һʱ����װ�� (��ܡ����ܡ�)�γ�ԭ��أ������ܣ���˵�����ɣ����ܣ���ָ����������
��3��ȼ�ϵ�صĹ���ԭ���ǽ�ȼ�Ϻ�������(��O2)��Ӧ���ų��Ļ�ѧ��ֱ��ת��Ϊ���ܡ������һȼ�ϵ�أ��Ե缫aΪ�������缫bΪ����������Ϊȼ�ϣ�����������ҺΪ���Һ��������Ӧͨ�� ��(��a��b����ͬ)�����Ӵ� ��������a�������缫��ӦʽΪ��_________________________
��1��2H++2e=H2��2�֣��ܣ�����þ�������� ��1��
��3��B��B ��1�� 4H+ �� O2 �� 4e�� �� 2H2O 2��

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�A. ������Һ�о�(ͼ1)���ڵ�����е�ǰ�ء�������Һ������̴ѡ�����Χ������FeS����ͭ��п��ȿ��
(1) Ni2���ĺ�������Ų�ʽ��____________________��
(2) �����±���ͭ�ĵ�һ������(I1)С��п�ĵ�һ�����ܣ���ͭ�ĵڶ�������(I2)ȴ����п�ĵڶ������ܣ�����Ҫԭ���ǡ���
����
| ������/kJ��mol��1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
(3) ����˵����ȷ����________��
A. �縺�ԣ�N��O��S��C����������������B. CO2��COS(����)��Ϊ�ȵ�����
C. NH3�����е�ԭ�Ӳ���sp3�ӻ� D. CO��H2S��HCN���Ǽ��Է���
(4) ��������Һ���д�������һ�������ӣ��ṹ��ͼ2�����Ļ�ѧʽΪ________________��
(5) FeS��NaCl��Ϊ���Ӿ��壬�������ƣ�ǰ���۵�Ϊ985�棬����801�棬��ԭ����____________________________________________����FeS�����У���Fe2����������������S2��Χ�ɵĶ�����Ŀռ乹��Ϊ________________��
B. �Ʊ�KNO3�����ʵ�������ýᾧ���ؽᾧ����KNO3��NaCl�Ļ������з��롣������ij��ѧ��ȤС��Ļ��¼��
|
| NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 20.9 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
�������ϣ������в�ã��������ڲ�ͬ�¶��µ��ܽ��(S/g)���±���
ʵ�鷽����
��. �ܽ⣺��ȡ29.8g KCl��34.0g NaNO3����250mL�ձ��У��ټ���70.0g����ˮ�����Ȳ����裬ʹ����ȫ���ܽ⡣
��. �����ᾧ���������Ⱥͽ��裬����Һ����Ũ������100��ʱ������50.0g ˮ��ά�ָ��¶ȣ��ڱ���©��(��ͼ��ʾ)�г��ȹ��������ľ��塣�þ���m1g��
��. ��ȴ�ᾧ������Һ��ȴ������(ʵ��ʱ����Ϊ10��)���м�ѹ���ˡ���KNO3�ֲ�Ʒm2g��
��. �ؽᾧ�����ֲ�Ʒȫ������ˮ���Ƴ�100��ı�����Һ����ȴ�����º���ˡ���KNO3��Ʒ��
�ٶ����� �����ʱ��Ӱ����Ե��ܽ�ȣ��� ���ֹ��˲��������У��ܼ�����ĺ��Բ��ơ��Իش��й����⣺
(1) �������г��ȹ��˵�Ŀ���ǡ���
(2) ���������гн���Һ���ձ��в���������ˮ�����������ڲ������пɵôֲ�Ʒ������m2��______g�����л���NaCl______g��Ϊ��ֹNaCl���룬�ڲ������гн���Һ���ձ�������Ӧ��������ˮ______g��
(3) �������в��ü�ѹ���ˣ����ŵ���______________________________________����С��ͬѧ���õ�װ������ͼ��ʾ����д����װ������Ҫ�õ��IJ������������ƣ�________________����ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ��______________________________________��
 ����
���� ����
����
 100��ı�����Һ����ȴ�����º���ˡ���KNO3��Ʒ��
100��ı�����Һ����ȴ�����º���ˡ���KNO3��Ʒ��
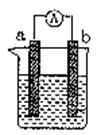
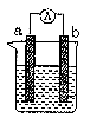 (1)���缫aΪAl���缫bΪCu���������ҺΪϡ����ʱ�������ĵ缫��ӦʽΪ��
(1)���缫aΪAl���缫bΪCu���������ҺΪϡ����ʱ�������ĵ缫��ӦʽΪ��