��Ŀ����
Ԫ��ͭ����Һ����Ҫ��[Cu(H2O)4]2+������ɫ����[Cu(OH)4]2-����ɫ����[CuCl2]-�� [Cu(NH3)4]2+������ɫ������ʽ���ڡ�CuClΪ������ˮ�İ�ɫ���塣�ش��������⣺
��1����Ũ��ǿ����Һ�У�Cu2+��Al3+�Ļ�ѧ�������ơ���������CuSO4��Һ�У�����Ũ��NaOH��Һֱ���������ɹ۲쵽��������_____________________��
��2��CuCl����Ũ����ʱ�����ķ�ӦΪCuCl+HCl H[CuCl2]��Ҫ��H[CuCl2]��Һ�����������ķ�����___________________�����ȵ�CuCl2��Һ�м���ͭ�ۣ����Ƶ�CuCl���䷴Ӧ�����ӷ���ʽΪ___________________����ʵ���ϸ÷�Ӧ���ѳ������У���ԭ����____________________��ʹ��Ӧ�����еIJ�����___________________��
H[CuCl2]��Ҫ��H[CuCl2]��Һ�����������ķ�����___________________�����ȵ�CuCl2��Һ�м���ͭ�ۣ����Ƶ�CuCl���䷴Ӧ�����ӷ���ʽΪ___________________����ʵ���ϸ÷�Ӧ���ѳ������У���ԭ����____________________��ʹ��Ӧ�����еIJ�����___________________��
��3��[Cu(H2O)4]2+��[Cu(NH3)4]2+����Һ�п����ת������t��ʱ����ʼŨ��Ϊ1mol•L-1��CuSO4��Һ��{ Cu2+ʵ����[Cu(H2O)4]2+����ʽ����}��[Cu(NH3)4]2+��Ũ���氱ˮŨ��[c(NH3)]�ı仯��ͼ��ʾ
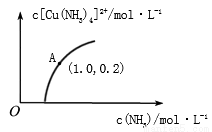
��CuSO4��Һ�������ˮ������Ӧ���ܵ����ӷ���ʽΪ________________��
����ͼ��֪����Һ����������[Cu(H2O)4]2+ ת��Ϊ[Cu(NH3)4]2+��ƽ��ת����___________���������С�����䡱��������A�����ݣ���������¶��µ�ƽ�ⳣ��K=__________��
�������¶ȣ���Һ��[Cu(H2O)4]2+��ƽ��ת���ʼ�С����÷�Ӧ�Ħ�H_____���>����<����=����0
���������Ⱥ�ͭ{[Cu(NH3)4]SO4}�ڼ��Զ�ͭ�����г��������Һ����Ҫ�ɷ֣����ʱ��Ҫ���ƾֲ��¶Ȳ��ø���150�棬���ܵ�ԭ����_______________�����ʱ�������ĵ缫��ӦʽΪ___________________��
H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
I.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
II.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ____________��
(3)��֪��25��ʱ��Ksp(SnS)=1.0��10-25��Ksp(CdS)=8.0��10-27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L-1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2+��ʼ����ʱ����Һ��c(Cd2+)=_________(��Һ����仯���Բ��ƣ���
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)+H2(g) H2S(g)+CO(g) ��H=+7 kJ��mol-1��
H2S(g)+CO(g) ��H=+7 kJ��mol-1��
��.CO(g)+H2O(g) CO2(g)+H2(g) ��H =-42 kJ��mol-1 ��
CO2(g)+H2(g) ��H =-42 kJ��mol-1 ��
(4)��֪������1 mol�����еĻ�ѧ���������յ��������±���ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/ kJ��mol-1 | 1319 | 442 | x | 678 | -930 | 1606 |
����x=___________��
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g),��������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶ȣ�T)�Ĺ�ϵ��ͼ��ʾ��

�������¶�����,CO��ƽ���������____________(�����С������ԭ��Ϊ_______________��
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶���,COS��ƽ��ת����Ϊ_________����Ӧi��ƽ�ⳣ��Ϊ____________(������λ��Ч����)��


 C(s)��D(g)����H>0�Ļ�ѧ��Ӧ����(v)��ʱ��(t)�Ĺ�ϵ��ͼ����ʾ���ǿ��淴Ӧ2NO2(g)
C(s)��D(g)����H>0�Ļ�ѧ��Ӧ����(v)��ʱ��(t)�Ĺ�ϵ��ͼ����ʾ���ǿ��淴Ӧ2NO2(g)
 xC(g) ��H��0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ1����ƽ�����t1��t2��t3��t4ʱ��ֻ�ı���һ���������淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ2
xC(g) ��H��0��B��C�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼ1����ƽ�����t1��t2��t3��t4ʱ��ֻ�ı���һ���������淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ2