��Ŀ����
���ֶ�����Ԫ��A��B��C��D�����ʻ�ṹ��Ϣ���£�
��Ϣ�� ԭ�Ӱ뾶��С��A>B>C>D
��Ϣ�� ����Ԫ��֮���γɵ�ij���ַ��ӵı���ģ�ͼ��������ʣ�

�����������Ϣ�ش��������⡣
��1��BԪ�������ڱ��е�λ�� ����д��BC2���ӵĵ���ʽ ��
��2��A���������У�EԪ�صĵ��ʻ�ԭ����ǿ��FԪ�ص�ijЩ���ೣ������ˮ����E�������Ӧ�л�����X���ɡ���д������������Ӧ�����ӷ���ʽ��
��AԪ�صĵ� �������ʼ����ķ�Ӧ ��
�������ʼ����ķ�Ӧ ��
��F��C����Ԫ����ɵĻ�����Y����Ϊ���½ṹ�մɲ��ϣ�X��Y���ֻ���������Һ�з����ķ�Ӧ ��
��3�����ʶ���Ԫ����ɺͼ���ͬ�������Ӿ���18���ӽṹ����ʢ��һ��Ũ�ȶ���Һ���Թ��У���μ���������ϡ�����ữ������������Һ���μӹ����е������� ��
��
��dz��ɫ��Һ������ػ�ɫ�������������ݳ��֣�����֤����������Ƭ�̺�Ӧ��þ��ң����ų��϶������������μ���Һ������һ��ʱ�䣬���Թ��г��ֺ��ɫ���������ٵ���ϡ���ᣬ���ɫ��Ϊ��ɫ�����û�ѧ����ʽ�����ӷ���ʽ�١��ڡ����мӵ���֡�
�� �� �� ��
�� ��
��Ϣ�� ԭ�Ӱ뾶��С��A>B>C>D
��Ϣ�� ����Ԫ��֮���γɵ�ij���ַ��ӵı���ģ�ͼ��������ʣ�

�����������Ϣ�ش��������⡣
��1��BԪ�������ڱ��е�λ�� ����д��BC2���ӵĵ���ʽ ��
��2��A���������У�EԪ�صĵ��ʻ�ԭ����ǿ��FԪ�ص�ijЩ���ೣ������ˮ����E�������Ӧ�л�����X���ɡ���д������������Ӧ�����ӷ���ʽ��
��AԪ�صĵ�
 �������ʼ����ķ�Ӧ ��
�������ʼ����ķ�Ӧ ����F��C����Ԫ����ɵĻ�����Y����Ϊ���½ṹ�մɲ��ϣ�X��Y���ֻ���������Һ�з����ķ�Ӧ ��
��3�����ʶ���Ԫ����ɺͼ���ͬ�������Ӿ���18���ӽṹ����ʢ��һ��Ũ�ȶ���Һ���Թ��У���μ���������ϡ�����ữ������������Һ���μӹ����е�������
 ��
����dz��ɫ��Һ������ػ�ɫ�������������ݳ��֣�����֤����������Ƭ�̺�Ӧ��þ��ң����ų��϶������������μ���Һ������һ��ʱ�䣬���Թ��г��ֺ��ɫ���������ٵ���ϡ���ᣬ���ɫ��Ϊ��ɫ�����û�ѧ����ʽ�����ӷ���ʽ�١��ڡ����мӵ���֡�
�� ��
 �� ��
�� ����1����2����IVA�� ��1�� ��
��2����Cl2+H2O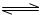 H++Cl-+HClO ��2�֣�
H++Cl-+HClO ��2�֣�
��Al2O3+2OH-===2AlO-2+H2O ��2�֣�
��3����2Fe2++H2O2+2H+=2Fe3++2H2O ��2�֣�
��2H2O2=2H2O+O2�� ��2�֣�
��Fe3++3H2O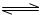 Fe��OH��3+3H+ ��2�֣���ע���٢�д��ѧ����ʽҲ���֣�
Fe��OH��3+3H+ ��2�֣���ע���٢�д��ѧ����ʽҲ���֣�
��2����Cl2+H2O
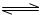 H++Cl-+HClO ��2�֣�
H++Cl-+HClO ��2�֣���Al2O3+2OH-===2AlO-2+H2O ��2�֣�
��3����2Fe2++H2O2+2H+=2Fe3++2H2O ��2�֣�
��2H2O2=2H2O+O2�� ��2�֣�
��Fe3++3H2O
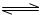 Fe��OH��3+3H+ ��2�֣���ע���٢�д��ѧ����ʽҲ���֣�
Fe��OH��3+3H+ ��2�֣���ע���٢�д��ѧ����ʽҲ���֣���

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
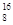 O��
O��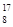 O��
O��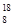 O��Ϊͬλ�أ���������Ԫ�أ���������ȫ��ͬ
O��Ϊͬλ�أ���������Ԫ�أ���������ȫ��ͬ C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��
C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��