��Ŀ����
����������ʾ���ʱ仯�ķ�Ӧʽ�У��������
| A��������ʴʱ���ܷ�����������Ӧ��2H2O + O2 + 4e��= 4OH�� |
| B����KHSO4��Һ�е���Ba(OH)2��ҺʹpH=7��SO42��+ 2 H+ + Ba2+ + 2OH��= BaSO4��+ 2 H2O |
C������ˮ������ӷ���ʽ��Al3+ + 3H2O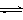 Al(OH)3 + 3H+ Al(OH)3 + 3H+ |
| D��K37ClO3��Ũ����(HCl)�ڼ���ʱ����������K37ClO3 + 6HCl��K37Cl + 3Cl2��+ 3H2O |
D
���������A��������ʴʱ���ܷ���������ʴ����������Ӧ��2H2O+O2+4e-=4OH-����A��ȷ��B����KHSO4��Һ�е���Ba��OH��2��ҺʹpH=7�����ӷ�ӦΪSO42-+2H++Ba2++2OH-=BaSO4��+2H2O����B��ȷ��C������ˮ������ӷ���ʽΪAl3++3H2O?Al��OH��3+3H+����C��ȷ��D��K37ClO3��Ũ���ᣨHCl���ڼ���ʱ���������ķ�ӦΪ2K37ClO3+12HCl
 2KCl+37Cl2��+5Cl2��+6H2O����D����
2KCl+37Cl2��+5Cl2��+6H2O����D����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
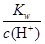 ��0.1 mol��L��1����Һ��Na����Cu2����HCO3����NO3��
��0.1 mol��L��1����Һ��Na����Cu2����HCO3����NO3��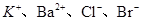
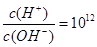 ����Һ�У�
����Һ�У� 

 NH3�� + H2O��NH3��ʹʪ��ĺ�ɫʯ����ֽ��������ij��Һ�п��ܺ�������6�������е�ij���֣�Na+��NH4+��K+��Cl����SO42����CO32����Ϊȷ����Һ��ɽ�������ʵ�飺��1��ȡ200 mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30g��������м�����������ᣬ��2.33g�������ܡ���2����1������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ����������1.12L���ѻ���ɱ�״�����ٶ�����������ȫ���ݳ������ɴ˿��Եó�����ԭ��Һ��ɵ���ȷ������
NH3�� + H2O��NH3��ʹʪ��ĺ�ɫʯ����ֽ��������ij��Һ�п��ܺ�������6�������е�ij���֣�Na+��NH4+��K+��Cl����SO42����CO32����Ϊȷ����Һ��ɽ�������ʵ�飺��1��ȡ200 mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30g��������м�����������ᣬ��2.33g�������ܡ���2����1������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ����������1.12L���ѻ���ɱ�״�����ٶ�����������ȫ���ݳ������ɴ˿��Եó�����ԭ��Һ��ɵ���ȷ������