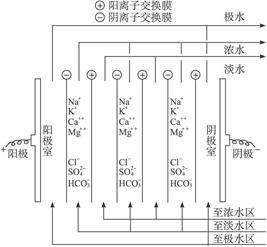
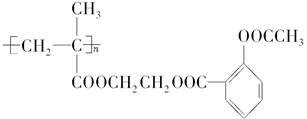��Ŀ����
��15�֣�Ϊ��ȥ�����е�Ca2+��Mg2+��Fe3+�� �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������


��1���ж�BaCl2�ѹ����ķ�����______________________________________��
��2���ڢܲ��У���ص����ӷ���ʽ��_____________________________________��
��3�������������pH�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
__________________________________________________��
��4��Ϊ���龫�δ��ȣ�������150 mL 0.2 mol��L-1 NaCl(���Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����______________________________________��
 �Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������
�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�������

��1���ж�BaCl2�ѹ����ķ�����______________________________________��
��2���ڢܲ��У���ص����ӷ���ʽ��_____________________________________��
��3�������������pH�ٹ��ˣ�����ʵ��������Ӱ�죬��ԭ����
__________________________________________________��
��4��Ϊ���龫�δ��ȣ�������150 mL 0.2 mol��L-1 NaCl(���Σ���Һ����ͼ�Ǹ�ͬѧת����Һ��ʾ��ͼ��ͼ�еĴ�����______________________________________��
��1��ȡ�ڢڲ�����ϲ���Һ1��2���ڵ�ΰ��ϣ��ٵ���1��2��BaCl2��Һ������Һδ����ǣ������BaCl2�ѹ���?
��2��Ca2++CO2-3====CaCO3��,Ba2++CO2-3====BaCO3��??
��3���ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ���?
��4��δ�ò�����������δ����150 mL����ƿ?
��2��Ca2++CO2-3====CaCO3��,Ba2++CO2-3====BaCO3��??
��3���ڴ���������£����в��ֳ����ܽ⣬�Ӷ�Ӱ���Ƶþ��εĴ���?
��4��δ�ò�����������δ����150 mL����ƿ?
��1������SO2-4�������Լ�Ϊ��Ba2+����Һ����ȡ�ڲ����ϲ���Һ�ټ�BaCl2�����Ƿ����������жϣ���û�а�ɫ����������֤��BaCl2�ѹ�����?
��2���ڢܲ���Ŀ����Ϊ�˳�ȥCa2+������Ba2+��������ӷ���ʽӦ��Ca2++CO2-3="===" CaCO3��,Ba2++CO2-3====BaCO3����
��3������ȼ������pH�ٹ��ˣ�����ʹ���ֳ����ܽ⣬��������ʳ�β�����?
��4������һ�����ʵ���Ũ����Һʱ��Ҫ����ѡ����ƿ���ݻ�Ӧ���������Һ�������ȣ���150 mL����ƿ��ת����ҺʱӦ�ò�������������ͼ����ʾû�а�Ҫ������
��2���ڢܲ���Ŀ����Ϊ�˳�ȥCa2+������Ba2+��������ӷ���ʽӦ��Ca2++CO2-3="===" CaCO3��,Ba2++CO2-3====BaCO3����
��3������ȼ������pH�ٹ��ˣ�����ʹ���ֳ����ܽ⣬��������ʳ�β�����?
��4������һ�����ʵ���Ũ����Һʱ��Ҫ����ѡ����ƿ���ݻ�Ӧ���������Һ�������ȣ���150 mL����ƿ��ת����ҺʱӦ�ò�������������ͼ����ʾû�а�Ҫ������

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д� ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
�����Ŀ

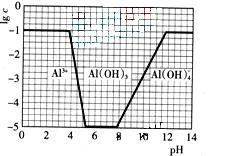
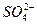 ��2 560 mg��L-1��Mg2+��1 272 mg��L-1��Ca2+��400 mg��L-1��K+��380 mg��L-1��HCO3����142 mg��L-1
��2 560 mg��L-1��Mg2+��1 272 mg��L-1��Ca2+��400 mg��L-1��K+��380 mg��L-1��HCO3����142 mg��L-1