��Ŀ����
������ȩͨ��Ϊ40%���ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ����ȩ����Һ�пɱ�������
��1��֤����Һ���Ƿ����в�����ȩ��������ʵ�������������
��2������������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ����û�ѧ����ʽ��ʾ��һ��Ӧ���̣�
��1��֤����Һ���Ƿ����в�����ȩ��������ʵ�������������
ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�����
ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�����
����2������������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ����û�ѧ����ʽ��ʾ��һ��Ӧ���̣�
CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O
CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O
����������1������ȩ��������������CH3COOH���²�Һ�����ԣ���������ʯ����Һ�����²�Һ�Ƿ�����ԣ�
��2����������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ���ɫ����Ϊ̼��Ũ�������ǿ�����ԣ�����������ȩ����C��ͬʱ���ɶ�������ˮ��
��2����������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ���ɫ����Ϊ̼��Ũ�������ǿ�����ԣ�����������ȩ����C��ͬʱ���ɶ�������ˮ��
����⣺��1������ȩ��������������CH3COOH���²�Һ�����ԣ���������ʯ����Һ�����²�Һ�Ƿ�����ԣ��������Ϊ��ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�������
�ʴ�Ϊ��ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�������
��2����������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ���ɫ����Ϊ̼��Ũ�������ǿ�����ԣ�����������ȩ����C��ͬʱ���ɶ�������ˮ����Ӧ����ʽΪ��CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O��
�ʴ�Ϊ��CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O��
�ʴ�Ϊ��ȡ�����²�ˮ��Һ���μ�ʯ����Һ�������Һ�ʺ�ɫ��˵��������ȩ�ѱ�������
��2����������ȩ��Һ�����ȵ�Ũ�����У��к�ɫ�����������ɣ���ɫ����Ϊ̼��Ũ�������ǿ�����ԣ�����������ȩ����C��ͬʱ���ɶ�������ˮ����Ӧ����ʽΪ��CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O��
�ʴ�Ϊ��CH3CHO+H2SO4��Ũ����2C��+SO2��+3H2O��
���������⿼��ȩ�����ʡ�ʵ�鷽����ơ���ѧ����ʽ����д�ȣ��ѶȲ���2���жϺ�ɫ����Ϊ̼������������ԭ��Ӧȷ��Ũ�������ǿ�����ԣ�

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ

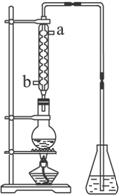


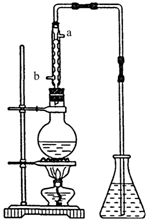 (4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�
(4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�