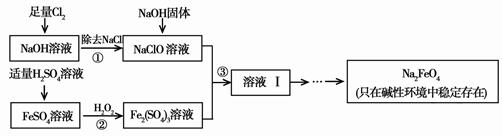
��Ŀ����
[2012����������һģ]��12�֣��������ƣ�Na2FeO4�����к�ǿ�������ԣ���һ�����͵���ɫ��ˮ����������ҵ�Ͽ���ͨ�����������������Ʊ��������ƣ������������£�

�ش��������⣺
��1����������ٺ���NaOH�����ԭ����____ ____��
��2������ڷ�Ӧ�����ӷ���ʽ��_____ ___��
��3������Һ���з����Na2FeO4���и���ƷNa2SO4 ��NaCl��������з�Ӧ�����ӷ���ʽΪ___ _____��
��4����һ������Na2FeO4Ͷ�뵽pH��ͬ����ˮ�У���ˮ������ɷ־���ͬ������Һ��Na2FeO4Ũ�ȱ仯����ͼ���ߢ���ʾ�����Ʋ�����I������II��Ӧ����ˮpH_____����ߡ��͡�����
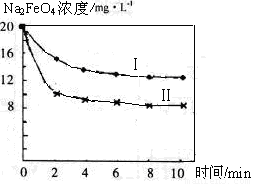
��5��ͨ�������֪Na2FeO4������Ч�ʣ��Ե�λ�����õ��ĵ�������ʾ����������_____����ߡ��͡������ø������ƴ�����������ˮ���������ŵ���____ _
��������㼴�ɣ���
��12�֣���1��Na2FeO4ֻ�ڼ��Ի������ȶ����ڣ����Լ����������ƿ��Ե�����Һ�Լ���
��2��2Fe2+��H2O2��2H+��2Fe3+��2H2O
��3��2Fe3+��3ClO����10OH����2FeO42����3Cl����5H2O
��4����
��5���� ��������ɱ�����ܾ�ˮ�������㱣��ȣ�
����������1�����ݿ�ͼ�е���ʾ��֪��Na2FeO4ֻ�ڼ��Ի������ȶ����ڣ����Լ����������ƿ��Ե�����Һ�Լ��ԡ�
��2�������������£�H2O2��Fe2+������Fe3+����������ԭ����H2O����Ӧ�����ӷ���ʽΪ2Fe2+��H2O2��2H+��2Fe3+��2H2O��
��3��������вμӷ�Ӧ��������Fe3+��ClO����OH����������FeO42����Cl�����H2O���ɣ��ʸ÷�Ӧ�����ӷ���ʽΪ2Fe3+��3ClO����10OH����2FeO42����3Cl����5H2O��
��4��Na2FeO4�ڼ��Ի������ȶ����ڣ���ˮ��pHԽ�ߣ�Na2FeO4��Ũ��Խ������I������II��Ӧ����ˮpH�ߡ�
��5��1gNa2FeO4�ܵõ����ӵ����ʵ���Ϊ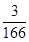 mol��0.018mol��1gCl2�ܵõ����ӵ����ʵ���Ϊ
mol��0.018mol��1gCl2�ܵõ����ӵ����ʵ���Ϊ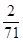 mol��0.028mol����Na2FeO4������Ч�ʱ������ĵ͡�
mol��0.028mol����Na2FeO4������Ч�ʱ������ĵ͡�