��Ŀ����
�������������������ش�������⡣
(1)ij��������������ķֲ������ͼ��ʾ��������W��X��Y��Z��ij��ˮ������������ʾ��

�ٵ���X��Y��ˮ��pH�仯��ԭ�������______________________________��
��Z������������٣��������������ԭ�������_________________________��
(2)ij������̽���̲��зḻ�ij�����(��Ҫ�ɷ�ΪFe2O3��������SiO2������)��ú��ʯ��ʯ����������ڸõ������������������

����������Ŀ������������Ľ�������Ҫ�ڸõ�����Ӧ���������������糧��ˮ�೧�ȣ��γɹ�ģ�Ĺ�ҵ��ϵ���ݴ�ȷ����ͼ����Ӧ����������A________��B________��C________��D________��
���Գ�����Ϊԭ�ϣ�д����¯�����еõ������Ͳ���¯���Ļ�ѧ����ʽ_____________________________________________________________��
�۴ӡ����ϡ����á����������ȽǶȿ��ǣ��õ�������ҵ��������Ӧ��ȡ��һЩ��ʩ��____________(�о�2�ּ���)��
(1)ij��������������ķֲ������ͼ��ʾ��������W��X��Y��Z��ij��ˮ������������ʾ��

| �ص� ��Ŀ | W | X | Y | Z |
| ˮ��/�� | 15 | 18 | 26 | 25 |
| pH | 6 | 8 | 5 | 5 |
| �ܽ�����/(mg��L��1) | 11 | 9 | 7 | 3 |
��Z������������٣��������������ԭ�������_________________________��
(2)ij������̽���̲��зḻ�ij�����(��Ҫ�ɷ�ΪFe2O3��������SiO2������)��ú��ʯ��ʯ����������ڸõ������������������

����������Ŀ������������Ľ�������Ҫ�ڸõ�����Ӧ���������������糧��ˮ�೧�ȣ��γɹ�ģ�Ĺ�ҵ��ϵ���ݴ�ȷ����ͼ����Ӧ����������A________��B________��C________��D________��
���Գ�����Ϊԭ�ϣ�д����¯�����еõ������Ͳ���¯���Ļ�ѧ����ʽ_____________________________________________________________��
�۴ӡ����ϡ����á����������ȽǶȿ��ǣ��õ�������ҵ��������Ӧ��ȡ��һЩ��ʩ��____________(�о�2�ּ���)��
(1)����ֽ���ŷŵļ�����ˮʹX����ˮpH���ߣ��������糧�������������Է�ˮ����δ�����ŷţ����Y���ĺ�ˮpH����(��������糧ȼ�ղ�����SO2�ᵼ�����꣬Ʈ���ʹY���ĺ�ˮpH����)
�ڻ��ʳ���ũ�P������ˮʹZ����ˮ��Ӫ������ˮ�½ϸߣ����������ˮ��ֲ����������ˮ���ܽ������������ģ�������������
(2)�ٷ��糧������������������ˮ�೧
��Fe2O3��3CO 2Fe��3CO2
2Fe��3CO2
CaCO3��SiO2 CaSiO3��CO2��
CaSiO3��CO2��
������������¯��(��CaSiO3)��Ϊˮ�೧��ԭ��
�÷��糧��ú��ʯ�ͷ�ú����Ϊˮ�೧��ԭ��
��ʯ��ʯ���ճ���ʯ�ң��������շ��糧�ͽ�����ȼúʱ������SO2�����ٶԿ�������Ⱦ������ˮ����ϵͳ
�ڻ��ʳ���ũ�P������ˮʹZ����ˮ��Ӫ������ˮ�½ϸߣ����������ˮ��ֲ����������ˮ���ܽ������������ģ�������������
(2)�ٷ��糧������������������ˮ�೧
��Fe2O3��3CO
 2Fe��3CO2
2Fe��3CO2CaCO3��SiO2
 CaSiO3��CO2��
CaSiO3��CO2��������������¯��(��CaSiO3)��Ϊˮ�೧��ԭ��
�÷��糧��ú��ʯ�ͷ�ú����Ϊˮ�೧��ԭ��
��ʯ��ʯ���ճ���ʯ�ң��������շ��糧�ͽ�����ȼúʱ������SO2�����ٶԿ�������Ⱦ������ˮ����ϵͳ
AΪ���糧����Ϊ������Ҫú̿������Ϊ���������ṩ���ܣ�BΪ��������C��ԭ��Ϊ�������̼��ƣ�̼��ƿ��ṩ������̼������ˮ����Ҫ�������ֽ���ŷŵļ�����ˮʹX����ˮpH���ߣ��������糧�������������Է�ˮ����δ�����ŷţ����Y���ĺ�ˮpH����(��������糧ȼ�ղ�����SO2�ᵼ�����꣬Ʈ���ʹY���ĺ�ˮpH����)�����ʳ���ũ�P������ˮʹZ����ˮ��Ӫ������ˮ�½ϸߣ����������ˮ��ֲ����������ˮ���ܽ������������ģ���������������

��ϰ��ϵ�д�
 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
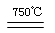 CaO��2HBr
CaO��2HBr HgBr2��H2��
HgBr2��H2��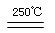 HgO��CaBr2
HgO��CaBr2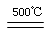 2Hg��O2��
2Hg��O2��