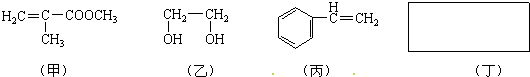
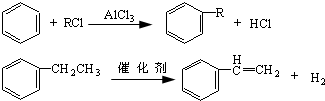
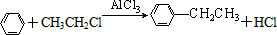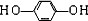��Ŀ����
�����������ֿ��������쵼�����״��ֺ��Աʹ�õ�����ƿ���Ʊ�����һ�ַ����к��������������ʣ�
����д���пհף�
(1)���в�����ԭ�ӵĹ������� ��
�����Լ������Ӧ����ɫ���� �����ţ�
| A��Br2/CCl4��Һ | B��ʯ����Һ | C������KMnO4��Һ | D������ |
(3)����ͨ������ת�����Եõ��ң�����A��D��Ϊ�л��:

A�Ļ�ѧʽ�� ��C��D�ķ�Ӧ������ ��
(4)�����ﶡ����̼���⡢������Ԫ�أ���Է�������Ϊ110������FeCl3��Һ��������ɫ���Ҷ������������ϵ�һ��ȡ����ֻ��һ�֡��Ľṹ��ʽΪ ��
(1)̼̼˫������ ����A��C
����A��C
(2)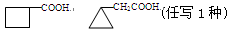
(3)C6H12O6���ӳɷ�Ӧ
(4)
����

��ϰ��ϵ�д�
�����Ŀ






 +CH3CH2Cl
+CH3CH2Cl +HCl
+HCl



 ��
��  ��������
��������