��Ŀ����
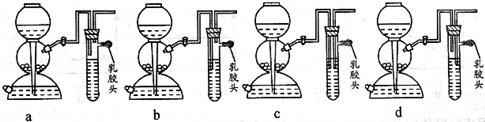
��1����ȼ�������������ܻᷢ����ը��Ϊ�˷�ֹ���⣬������һ����ȫװ�ã���ͼ��װ���������õ���______��

��2��ȡһ�����ı�����ˮ��CaCO3��ĩ��ϣ��۲쵽�������ݣ���ˮ�Ļ���ɫ��ȥ����ֹ��ȡ�ϲ������Һ�ķݣ��ֱ��������ʵ�飺
��һ�ݵμ����ᣬ������������
��һ�ݵμ�NaOH��Һ�����ְ�ɫ����
��һ���þƾ��Ƽ��ȳ��ְ�ɫ����
�ܽ���ɫ����������ķ���Һ������������ɫ
������ʵ�������Ʋ�ó�����Һ����Ҫ������______��ʵ��ٿ�����______����ʽ��ʾ��������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽ______��
�⣺��1��a�����巢��װ�������װ�ò��ֿܷ������κ����ã���a����
b����ȫװ��Ӧ���ֿܷ����巢��װ�������װ�ã���ֹ��ը���Ҳ�Ӱ������ĵ����ͼ��飬��b��ȷ��
c����װ����������������c����
d����װ����������������d����
�ʴ�Ϊ��b��
��2��������ˮ��Ӧ��������ʹ����ᣬ���������Ա�̼������
��һ�ݵμ����ᣬ�����������ݣ�˵������Һ�к���Ca��HCO3��2��
��һ�ݵμ�NaOH��Һ�����ְ�ɫ��������ӦΪCa��HCO3��2��NaOH��Ӧ����CaCO3��
��һ���þƾ��Ƽ��ȳ��ְ�ɫ������Ca��HCO3��2���ȷֽ�����CaCO3��
�ܽ���ɫ����������ķ���Һ������������ɫ��˵����Һ�к���HClO��
���ԣ��ó�����Һ����Ҫ������Ca��HCO3��2��CaCl2��HClO��
�������ᷴӦ�Ļ�ѧ����ʽΪCa��HCO3��2+2HCl=CaCl2+2CO2+2H2O��
������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽΪ2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
�ʴ�Ϊ��Ca��HCO3��2��CaCl2��HClO��Ca��HCO3��2+2HCl=CaCl2+CO2+2H2O��
2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
��������1����Ϊ��ȼ�������������ϵ�ȼ�ױ�ը����ν��ȫװ��Ӧ���ܽ����巢��װ�������װ�÷ֿ����Ҳ�Ӱ������ļ���ģ�
��2��������ˮ��Ӧ��������ʹ����ᣬ���������Ա�̼����������̼��Ʒ�ĩ�μ����������������˵����Һ�к���Ca��HCO3��2��
��Ӧ����ʽΪ2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
���������⿼��������Ʊ�����ˮ�����ʣ���Ŀ�Ѷ��еȣ������״���Ϊ��1����ע�ⰲȫƿ�����ã�
b����ȫװ��Ӧ���ֿܷ����巢��װ�������װ�ã���ֹ��ը���Ҳ�Ӱ������ĵ����ͼ��飬��b��ȷ��
c����װ����������������c����
d����װ����������������d����
�ʴ�Ϊ��b��
��2��������ˮ��Ӧ��������ʹ����ᣬ���������Ա�̼������
��һ�ݵμ����ᣬ�����������ݣ�˵������Һ�к���Ca��HCO3��2��
��һ�ݵμ�NaOH��Һ�����ְ�ɫ��������ӦΪCa��HCO3��2��NaOH��Ӧ����CaCO3��
��һ���þƾ��Ƽ��ȳ��ְ�ɫ������Ca��HCO3��2���ȷֽ�����CaCO3��
�ܽ���ɫ����������ķ���Һ������������ɫ��˵����Һ�к���HClO��
���ԣ��ó�����Һ����Ҫ������Ca��HCO3��2��CaCl2��HClO��
�������ᷴӦ�Ļ�ѧ����ʽΪCa��HCO3��2+2HCl=CaCl2+2CO2+2H2O��
������ˮ��CaCO3��ĩ������Ӧ�Ļ�ѧ����ʽΪ2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
�ʴ�Ϊ��Ca��HCO3��2��CaCl2��HClO��Ca��HCO3��2+2HCl=CaCl2+CO2+2H2O��
2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
��������1����Ϊ��ȼ�������������ϵ�ȼ�ױ�ը����ν��ȫװ��Ӧ���ܽ����巢��װ�������װ�÷ֿ����Ҳ�Ӱ������ļ���ģ�
��2��������ˮ��Ӧ��������ʹ����ᣬ���������Ա�̼����������̼��Ʒ�ĩ�μ����������������˵����Һ�к���Ca��HCO3��2��
��Ӧ����ʽΪ2CaCO3+2Cl2+2H2O=Ca��HCO3��2+CaCl2+2HClO��
���������⿼��������Ʊ�����ˮ�����ʣ���Ŀ�Ѷ��еȣ������״���Ϊ��1����ע�ⰲȫƿ�����ã�

��ϰ��ϵ�д�
�����Ŀ

 ��
��
