��Ŀ����
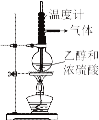
����Ŀ��1911�꣬��ѧ�ҷ��ֹ���4.2K����ʱ����ͻȻ�����������������³����ԡ�1986�꣬��ѧ���ַ�����Nb3Ge��23K�¾��г����ԡ�1987��2�£������ͼ������߶�����������Һ������(�е�77 K)�ĸ��³����壬�侧����ͼ��ʾ��Ԫ�����ΪBa-Y-Cu-O(�ٽ��¶�93 K)���ƶ��˹��ʸ��³����о���������Ժʿ���2016��ȹ�����߿�ѧ��������

�ش��������⣺
(1)��Nbλ�ڵ������ڣ�Nb����Χ�����Ų�ʽΪ4d45s1��Nbλ��_______�塣
(2)���й���GeԪ��������ȷ����______(������ѡ����ѡ��)��
A��Ge����������������Ϊԭ�Ӿ��� B��Ge����p���Ĺ��ɽ���
C��Ge�ĵ�һ�����ܱ�As��Se��ҪС D��Ge�ĵ縺�Ա�C��
(3)Ge(CH3)2Cl2���ӵ�����ԭ��Ge���ӻ���ʽ��______________��
(4)NH3Ҳ����������������_______(��������������С����)109��28������Ҫԭ����___________________________________________________________________________��
(5)ͼʾ���ϵ����뻯ѧʽ(��λʱ)Ϊ___________________����Y(��)Ԫ�صĻ��ϼ�Ϊ+3����Cu��ƽ�����ϼ�Ϊ__________��
���𰸡���B AC sp3 С�� ����������Nԭ���γ�3��N-H��������1�Թµ��Ӷԣ�����Nԭ�Ӳ�ȡsp3�ӻ�������ӹ���Ϊ������ Ba2YCu3O9 +![]()
��������
(1)��Nbλ�ڵ������ڣ�Nb����Χ�����Ų�ʽΪ4d45s1�������ڱ���ds���������Χ�����Ų�ʽ�����жϣ�
(2)Ge���ڵ������ڵ�IVA�壬���ڽ�����ǽ������紦������һ���Ľ�������ǽ����ԣ�
(3)Geԭ����������ȫ������ɼ���û�йµ��Ӷԣ��γ�4��������
(4)����������Nԭ���γ�3��N-H��������1�Թµ��Ӷԣ����ݷ��ӹ��ͷ����жϣ�
(5)��̯������ṹ��Ԫ�и�ԭ����Ŀ��ȷ����ѧʽ����ϻ��ϼ۹���ȷ��Cu��ƽ�����ϼۡ�
(1)��Nbλ�ڵ������ڣ�Nb����Χ�����Ų�ʽΪ4d45s1�������ڱ���ds����4d����4�����ӣ�5s����һ�����ӣ��ڢ�B�壻
(2)Ge���ڵ������ڵ�IVA�壬����p��Ԫ�أ����ڽ�����ǽ������紦������һ���Ľ�������ǽ����ԣ������������������
A��Ge���ڵ������ڵ�IVA�壬���ڽ�����ǽ������紦��Ge����������������Ϊԭ�Ӿ��壬��A��ȷ��
B��Ge����p���������ǹ��ɽ�������B����
C��ͬ����Ԫ�ش������ҵ�һ������������As��4p�ܼ��Ϻ���3�����ӣ�Ϊ������ȶ�״̬����һ�����ܽϸߣ����һ�����ܱ�As��Se��Ge����C��ȷ��
D��ͬ������ϵ��·ǽ����Լ������縺�Լ�������Ge�ĵ縺�Ա�CС����D����
��ѡAC��
(3)Geԭ����������ȫ������ɼ���û�йµ��Ӷԣ��γ�4���������ӻ������ĿΪ4��Geԭ�Ӳ�ȡsp3�ӻ���
(4)NH3Ҳ���������������������Nԭ���γ�3��N-H��������1�Թµ��Ӷԣ�����Nԭ�Ӳ�ȡsp3�ӻ�������ӹ���Ϊ�����Σ������С��109��28����
(5)Ba��Y���ڽṹ��Ԫ�ڲ����ṹ��Ԫ��Baԭ����Ŀ=2��Yԭ����Ŀ=1��Cuԭ�Ӵ��ڽṹ��Ԫ�Ķ��㡢���ϣ�Cuԭ����Ŀ=8��![]() +8��
+8��![]() =3��Oԭ�Ӵ������ϡ����ϣ�Oԭ����Ŀ=20��
=3��Oԭ�Ӵ������ϡ����ϣ�Oԭ����Ŀ=20��![]() +8��
+8��![]() =9���ʻ�ѧʽΪ��Ba2YCu3O9����Cu��ƽ�����ϼ�Ϊa����3a+2��(+2)+3+9��(2)=0�����a=+
=9���ʻ�ѧʽΪ��Ba2YCu3O9����Cu��ƽ�����ϼ�Ϊa����3a+2��(+2)+3+9��(2)=0�����a=+![]() ��
��

����Ŀ������a��b��c��d��Ϊ��ѧ��ѧ�еij������ʻ������֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ�������и��������У�ͨ��һ����Ӧ����ʵ��ͼʾת������
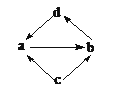
ѡ�� | a | b | c | d |
A | SO2 | SO3 | S | H2SO4 |
B | Na2O | Na2O2 | Na | NaOH |
C | CO | CO2 | C | H2CO3 |
D | Al2O3 | NaAlO2 | Al | Al(OH)3 |
A.AB.BC.CD.D