题目内容
(4分)氢气和氧气反应生成1mol水蒸气放热241.8KJ,已知氢气中H-H键能为436kJ/mol,氧气分子O=O键能为498 kJ/mol,则水分子中O-H键的键能为 kJ/mol 。若1 g水蒸气转化成液态水时放热2.5 kJ,则反应H2(g) + 1/2O2(g) == H2O(l)的△H= kJ/mol
(4分)463.4 kJ/mol 286.8 kJ/mol
△H=反应物键能之和—生成物键能之和=436+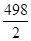 —O-H键的键能×2=—241.8
—O-H键的键能×2=—241.8
得O-H键的键能为463.4kJ/mol
由题意知②H2(g)+1/2O2(g)=H2O(g) △H=-241.8K+·mol-1
②H2O(g)=H2O(l) △H=-2.5×18kJ·mol-1
将①+②式得:H2(g)+1/2O2(g)=H2O(l) △H=-286.8KJ·mol-1
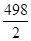 —O-H键的键能×2=—241.8
—O-H键的键能×2=—241.8得O-H键的键能为463.4kJ/mol
由题意知②H2(g)+1/2O2(g)=H2O(g) △H=-241.8K+·mol-1
②H2O(g)=H2O(l) △H=-2.5×18kJ·mol-1
将①+②式得:H2(g)+1/2O2(g)=H2O(l) △H=-286.8KJ·mol-1

练习册系列答案
相关题目
 2CO2(g);△H=-566kJ/mol
2CO2(g);△H=-566kJ/mol  CO(g)+3H2(g)……Ⅰ
CO(g)+3H2(g)……Ⅰ
 CH3OH(g)……Ⅱ
CH3OH(g)……Ⅱ
 ,若向一密闭容器中加入1mol
,若向一密闭容器中加入1mol 和0.5mol
和0.5mol 充分反应后,放出的热量为0.5
充分反应后,放出的热量为0.5
