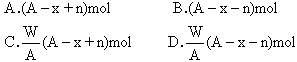��Ŀ����
ijԪ�ص�һ��ͬλ��R�����������ԣ���ԭ�Ӻ�������������������43��������ɵĹ��嵥��A����һ���������ܶ�Ϊ6.88 g/m3����X�����о������������ڱ߳�Ϊ1.00��10��7 cm���������к���20��ԭ�ӡ�
R�ڻ�ѧ��Ӧ�г�����Ϊ+2��+4�ۣ�����Һ��R2+�ȶ�����R4+��ǿ�����ԣ��ɽ�NO����ΪHNO3���ش�
��1��R��Ħ������Ϊ__________��
��2��R�������ڱ���__________����__________�塣
��3������R4+����Һ���뵽Fe��NO3��2��Һ�У���Ӧ�����ӷ���ʽΪ________________��
��1��207 g/mol
��2���� ��A
��3��Pb4++2Fe2+====Pb2++2Fe3+
����:
(20/NA)M/(1.00��10��7 cm)3=6.88 g/m3,���M=207 g/mol��

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ