��Ŀ����
ȡ(1)
(2)�����ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��___________��ԭ����_________________________��
(3)������ö�Ԫ��������һ���ǻ�����һ��±ԭ�ӣ����õ���±����ֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��_____________________________________________________��
������(1)������ɽ����Ͷ�Ԫ���ķ���ʽд��CxHy(H2O)z��
���������ʵ�ȼ�գ�
Cx��xC+xO2![]() xCO2(�������)
xCO2(�������)
Hy��yH+![]() O2
O2![]()
![]() H2O(l)(�����С)
H2O(l)(�����С)
(H2O)z��������������仯�ء�
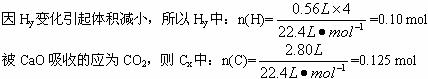
![]()
�ɵ�n(C)=0.125 mol
n(H)=0.10 mol+0.10 mol��2=0.30 mol
n(O)=0.10 mol
n(C)��n(H)��n(O)=0.125��0.30��0.10=5��12��4��
(2)�ô�ʵ��ʽΪC5H12O4��ʽ��Hԭ���ѱ��ͣ��������������Ǵ��ķ���ʽ��(3)�����⣬C5H12O4Ϊ��Ԫ�������ǻ�������λ���ǵ�ͬ�ģ�����Ҫ���ֻ�м����Ĵ�C(CH2OH)4��
�𰸣�(1)0.1250.3000.1005��12��4
(2)���� ��Ϊ�ô�ʵ��ʽ��Hԭ�Ӹ����ѱ��ͣ�����ʵ��ʽ������ʽC5H12O4
(3)C(CH2OH)4

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�