��Ŀ����
ij�����о�С�飬�ú��н϶����ʵ�ͭ����ȡ���̣�����Ƶ�ʵ��������£�
��1��ͭ�к��д������л���ɲ������յķ�����ȥ�л������ʱ������������
A�������� B��ʯ����C��������D��������E������ǯF���Թܼ�
��2��ͬ��������ͭ��ȡ������������е�ʵ��������裺���ܡ����ȡ�ͨ���������ˡ�
��3���ڲⶨ���õ���[CuSO4?xH2O]�нᾧˮxֵ��ʵ������У������������ٽ���
��������1������ʱ�������������������ϣ�ȡ������ʹ������ǯ�����պ������Ӧ����ʯ�����ϣ�
��2�����˺���ҺΪ����ͭ��Һ��Ȼ���������ᾧ�õ����壬���˺�ϴ�Ӹ��
��3���ⶨ���õ���[CuSO4?xH2O]�нᾧˮxֵ��ʵ������У���Ҫ�ⶨ����������ʧȥ�ᾧˮ�������������������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ��������������
��2�����˺���ҺΪ����ͭ��Һ��Ȼ���������ᾧ�õ����壬���˺�ϴ�Ӹ��
��3���ⶨ���õ���[CuSO4?xH2O]�нᾧˮxֵ��ʵ������У���Ҫ�ⶨ����������ʧȥ�ᾧˮ�������������������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ��������������
����⣺��1�����������ȹ���ʱӦ�����������������ϼ��ȣ�����ʱ������ǯ�г����������Ⱥ����ʯ��������ȴ���ʴ�Ϊ��C��E��B��
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壬�ʴ�Ϊ�����������ˣ�
��3���ⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ������������Χ�ڼ�����ֵ�Ƿ�������0.1g��������4�Σ��ʴ�Ϊ��4��
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壬�ʴ�Ϊ�����������ˣ�
��3���ⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ������������Χ�ڼ�����ֵ�Ƿ�������0.1g��������4�Σ��ʴ�Ϊ��4��
���������⿼������ͭ������Ʊ�������ƣ��漰��ѧʵ���������������ʵ��ԭ�����ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
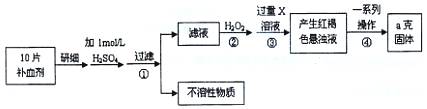
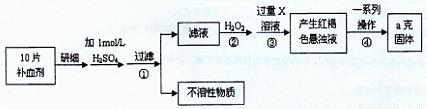
�����Ŀ
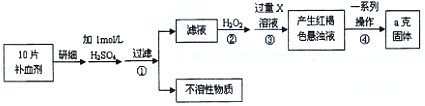

 ��ش��������⣺
��ش��������⣺ ��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��