��Ŀ����
�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6����
��1����������ɵñ�ϩ��
��֪��C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
����ͬ������ӦC3H8��g����CH3CH=CH2��g��+H2��g���ġ�H=
��2������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ������0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ���������൱��25�桢101kPa�²�õ���������
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ
������֪H2O��l��=H2O��g����H=+44kJ/mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
�۴˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ���
��1����������ɵñ�ϩ��
��֪��C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
����ͬ������ӦC3H8��g����CH3CH=CH2��g��+H2��g���ġ�H=
124.2
124.2
kJ?mol-1��2������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ������0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ���������൱��25�桢101kPa�²�õ���������
�ٷ�Ӧ���Ȼ�ѧ����ʽΪ
N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640kJ?mol-1
N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640kJ?mol-1
��������֪H2O��l��=H2O��g����H=+44kJ/mol����16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������
404
404
kJ���۴˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ���
���ﲻ����ɻ�����Ⱦ
���ﲻ����ɻ�����Ⱦ
����������1�����ݸ�˹���ɽ���Ȼ�ѧ����ʽ����õ���
��2���ٰ�0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ��������д����ѧ����ʽ������1mol�·�Ӧ���ȣ������Ȼ�ѧ����ʽ��д����д����
�����ݸ�˹���ɽ���Ȼ�ѧ����ʽ����õ���
�����ݷ�Ӧ��������жϣ�
��2���ٰ�0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ��������д����ѧ����ʽ������1mol�·�Ӧ���ȣ������Ȼ�ѧ����ʽ��д����д����
�����ݸ�˹���ɽ���Ȼ�ѧ����ʽ����õ���
�����ݷ�Ӧ��������жϣ�
����⣺��1����C3H8��g����CH4��g��+HC��CH��g��+H2��g����H1=156.6kJ?mol-1
��CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ���C3H8��g����CH3CH=CH2��g��+H2��g����H=124.2KJ/mol��
�ʴ�Ϊ��124.2��
��2���ٰ�0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ��������д����ѧ����ʽ������1mol�·�Ӧ����640KJ�������Ȼ�ѧ����ʽ��д����д���Ļ�ѧ����ʽΪ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1��
�ʴ�Ϊ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1��
�ڢ�N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1
��H2O��l��=H2O��g����H=+44kJ/mol
���ݸ�˹���ɢ�-4���ڵõ�N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-816kJ?mol-1
�����Ȼ�ѧ����ʽ���㣬16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������404kJ��
�ʴ�Ϊ��404��
�۲����ǵ�����ˮ�����ɲ�������Ⱦ���ʴ�Ϊ�����ﲻ����ɻ�����Ⱦ��
��CH3CH=CH2��g����CH4��g��+HC��CH��g����H2=32.4kJ?mol-1
���ݸ�˹���ɢ�-�ڵõ���C3H8��g����CH3CH=CH2��g��+H2��g����H=124.2KJ/mol��
�ʴ�Ϊ��124.2��
��2���ٰ�0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ��������д����ѧ����ʽ������1mol�·�Ӧ����640KJ�������Ȼ�ѧ����ʽ��д����д���Ļ�ѧ����ʽΪ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1��
�ʴ�Ϊ��N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1��
�ڢ�N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-640 kJ?mol-1
��H2O��l��=H2O��g����H=+44kJ/mol
���ݸ�˹���ɢ�-4���ڵõ�N2H4��l��+2H2O2��l��=N2��g��+4H2O��g����H=-816kJ?mol-1
�����Ȼ�ѧ����ʽ���㣬16gҺ̬����Һ̬˫��ˮ��Ӧ����Һ̬ˮʱ�ų���������404kJ��
�ʴ�Ϊ��404��
�۲����ǵ�����ˮ�����ɲ�������Ⱦ���ʴ�Ϊ�����ﲻ����ɻ�����Ⱦ��
���������⿼���Ȼ�ѧ����ʽ��д����˹���ɵļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ
 HCO3-+H+��ƽ�ⳣ��K1=
HCO3-+H+��ƽ�ⳣ��K1= CO32-+H+��HCO3-+H2O
CO32-+H+��HCO3-+H2O H2CO3+OH-��
H2CO3+OH-��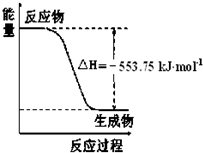 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���