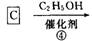��Ŀ����
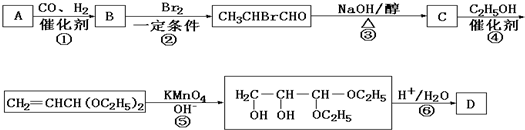
������A��ʯ�ͻ�����һ����Ҫԭ�ϣ���A��ˮú��Ϊԭ�Ͼ�����;���ϳɻ�����D������ʽΪC3H6O3����
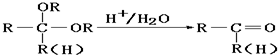
��֪��
��ش��������⣺
��1��д���������ʵĽṹ��ʽ��
A��______��B��______��C��______��D��______��
��2��ָ����Ӧ�ڵķ�Ӧ����______��
��3��д����Ӧ�۵Ļ�ѧ����ʽ______��
��4����Ӧ�ܵ�Ŀ����______��
��5��������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ���������ԭ�ӻ�״������F��C6H8O4������ֱ�д��D������E��F�Ļ�ѧ����ʽ��
D����E��______��
D����F��______��
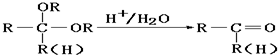
��֪��

��ش��������⣺
��1��д���������ʵĽṹ��ʽ��
A��______��B��______��C��______��D��______��
��2��ָ����Ӧ�ڵķ�Ӧ����______��
��3��д����Ӧ�۵Ļ�ѧ����ʽ______��
��4����Ӧ�ܵ�Ŀ����______��
��5��������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ���������ԭ�ӻ�״������F��C6H8O4������ֱ�д��D������E��F�Ļ�ѧ����ʽ��
D����E��______��
D����F��______��
�ɷ�Ӧ��B+Br2��CH3CHBrCHO��֪���÷�ӦΪȡ����Ӧ����BΪCH3CH2CHO���ɷ�Ӧ�ٿ�֪��AΪCH2=CH2���ɷ�Ӧ��CH3CHBrCHO
C���÷�ӦΪ����±��������ȥ��Ӧ����CΪCH2=CHCHO����Ӧ���ڸ�����ؼ�����Һ����C=C˫��������2��-OH����Ӧ�ܵ������DZ���ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ����������Ϣ��֪����Ӧ��Ϊ�Ѽ�����ˮ�ⷴӦ����������ȩ��-CHO����DΪCH2��OH��CH��OH��CHO��
��1��������������֪��AΪCH2=CH2��BΪCH3CH2CHO��CΪCH2=CHCHO��DΪCH2��OH��CH��OH��CHO��
�ʴ�Ϊ��CH2=CH2��CH3CH2CHO��CH2=CHCHO��CH2��OH��CH��OH��CHO��
��2����Ӧ����CH3CHBrCHOһ�����������巢��ȡ����Ӧ������CH3CHBrCHO��
�ʴ�Ϊ��ȡ����Ӧ��
��3����Ӧ����CH3CHBrCHO���������ƴ���Һ�����������·�����ȥ��Ӧ����CH2=CHCHO����Ӧ����ʽΪ��CH3CHBrCHO+NaOH
CH2=CHCHO+NaBr+H2O��
�ʴ�Ϊ��CH3CHBrCHO+NaOH
CH2=CHCHO+NaBr+H2O��
��4����Ӧ���ڸ�����ؼ�����Һ����C=C˫��������2��-OH����Ӧ�ܵ������DZ���ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ������
�ʴ�Ϊ������ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ������
��5��������D����CH2��OH��CH��OH��CHO��һ��ͬ���칹�壬D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ���������ԭ�ӻ�״������F��C6H8O4������D���к����ǻ�-OH���Ȼ�-COOH�������緢������ţ���У��������������л���м�����D��ΪCH3CH��OH��COOH��EΪCH2�TCHCOOH��FΪ
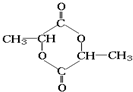
D���E�ķ�Ӧ����ʽΪ��CH3CH��OH��COOH
CH2=CHCOOH+H2O��
D���F�ķ�Ӧ����ʽΪ��
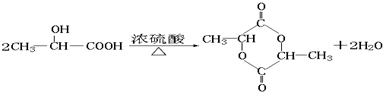
��
�ʴ�Ϊ��CH3CH��OH��COOH
CH2=CHCOOH+H2O��

��
| NaOH�� |
| �� |
��1��������������֪��AΪCH2=CH2��BΪCH3CH2CHO��CΪCH2=CHCHO��DΪCH2��OH��CH��OH��CHO��
�ʴ�Ϊ��CH2=CH2��CH3CH2CHO��CH2=CHCHO��CH2��OH��CH��OH��CHO��
��2����Ӧ����CH3CHBrCHOһ�����������巢��ȡ����Ӧ������CH3CHBrCHO��
�ʴ�Ϊ��ȡ����Ӧ��
��3����Ӧ����CH3CHBrCHO���������ƴ���Һ�����������·�����ȥ��Ӧ����CH2=CHCHO����Ӧ����ʽΪ��CH3CHBrCHO+NaOH
| �� |
| �� |
�ʴ�Ϊ��CH3CHBrCHO+NaOH
| �� |
| �� |
��4����Ӧ���ڸ�����ؼ�����Һ����C=C˫��������2��-OH����Ӧ�ܵ������DZ���ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ������
�ʴ�Ϊ������ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ������
��5��������D����CH2��OH��CH��OH��CHO��һ��ͬ���칹�壬D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ���������ԭ�ӻ�״������F��C6H8O4������D���к����ǻ�-OH���Ȼ�-COOH�������緢������ţ���У��������������л���м�����D��ΪCH3CH��OH��COOH��EΪCH2�TCHCOOH��FΪ

D���E�ķ�Ӧ����ʽΪ��CH3CH��OH��COOH
| Ũ���� |
| �� |
D���F�ķ�Ӧ����ʽΪ��

��
�ʴ�Ϊ��CH3CH��OH��COOH
| Ũ���� |
| �� |

��

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�
�����Ŀ