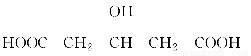ΧβΡΩΡΎ»ί
AΓΔBΓΔCΈΣΕΧ÷ήΤΎ‘ΣΥΊΘ§‘Ύ÷ήΤΎ±μ÷–Υυ¥ΠΒΡΈΜ÷Ο»γΆΦΥυ ΨΓΘAΓΔCΝΫ‘ΣΥΊΒΡ‘≠Ή”ΚΥΆβΒγΉ” ΐ÷°ΚΆΒ»”ΎB‘≠Ή”ΒΡ÷ Ή” ΐΓΘB‘≠Ή”ΚΥΡΎ÷ Ή” ΐΚΆ÷–Ή” ΐœύΒ»ΓΘ
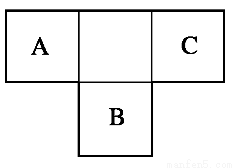
Θ®1Θ©AΓΔBΓΔC»ΐ÷÷‘ΣΥΊΒΡΟϊ≥ΤΖ÷±πΈΣ__________ΓΔ________ΓΔ________ΓΘ
Θ®2Θ©BΈΜ”Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎ________÷ήΤΎΒΎ________ΉεΓΘ
Θ®3Θ©CΒΡ‘≠Ή”ΫαΙΙ Ψ“βΆΦΈΣ________Θ§CΒΡΒΞ÷ ”κH2Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_______________________________________________________________________ΓΘ
Θ®4Θ©–¥≥ωAΒΡΤχΧ§«βΜ·Έο”κBΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ__________________________________________________________ΓΘ
Θ®1Θ©ΒΣ Νρ Ζζ
Θ®2Θ©»ΐ ΔωA
Θ®3Θ©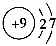 F2ΘΪH2=2HF
F2ΘΪH2=2HF
Θ®4Θ©2NH3ΘΪH2SO4=Θ®NH4Θ©2SO4
ΓΨΫβΈωΓΩ“άΧβ“βΘ§AΓΔBΓΔCΈΣΕΧ÷ήΤΎ‘ΣΥΊΘ§¥”AΓΔBΓΔCΒΡœύΕ‘ΈΜ÷ΟΩ¥Θ§AΓΔC÷ΜΡή¥Π‘ΎΒΎΕΰ÷ήΤΎΘ§ΕχB¥Π‘ΎΒΎ»ΐ÷ήΤΎΓΘ…ηAΒΡ‘≠Ή”–ρ ΐΈΣxΘ≠1Θ§‘ρCΈΣxΘΪ1Θ§BΈΣxΘΪ8Θ§‘ρ”–ΘΚΘ®xΘ≠1Θ©ΘΪxΘΪ1ΘΫxΘΪ8Θ§ΒΟxΘΫ8Θ§Υυ“‘AΓΔBΓΔCΒΡ‘≠Ή”–ρ ΐΖ÷±πΈΣ7ΓΔ16ΓΔ9Θ§Ε‘”ΠΒΡ‘ΣΥΊΖ÷±πΈΣNΓΔSΓΔFΓΘ