��Ŀ����
����Ŀ����֪Ca(OH)2��Cl2��Ӧ�����������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl-��ClO-��ClO3-���ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-�������ӵ����ʵ���(n)�뷴Ӧʱ��(t)������
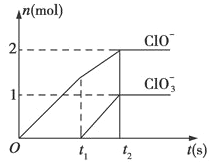
��1��t1ǰ������������___ ___(�ѧʽ)��
��2��t2ʱ��ʯ������Cl2������Ӧ���ܵ����ӷ���ʽΪ��____ __��
��3���÷�Ӧ�����������������ʵ�����______mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ��������__________(����ĸ)��
A��NaCl��Cl2 B��NaCl��NaClO C��NaClO3��NaClO4 D��NaCl��NaClO3
��5����ƽ�������ӷ���ʽ��
_______Fe(OH)3+_______ClO-+_______OH-=_______FeO42-+_______Cl-+_______H2O
���𰸡���1��Ca(ClO)2
��2��5Ca(OH)2+5Cl2=5Ca2++7Cl-+2ClO-+ClO3-+5H2O
��3��5��4��D��5��2Fe(OH)3��3ClO-+4OH-=2FeO42-+3Cl-+5H2O
��������
�����������1��������ԭ��Ӧ�ڵ����������ǻ�ԭ�����������ɵ����ʣ����ϼ��ڱ仯�����ߣ�����Ԫ�ػ��ϼ��������ɵIJ�����ͼ�������t1ǰ����������ֻ��Ca(ClO)2��
��2��t2ʱ��Ca(OH)2��Cl2������Ӧ������ͼ�������֪���ɴ��������������������ʵ���֮��Ϊ2��1�������������������ӷ���ʽ����дԭ�����غ㡢ԭ���غ���ƽ����ʽ��
5Ca(OH)2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O��
��3��t2ʱ���������ƺ�����ǡ�÷�Ӧ�����ݷ�Ӧ�����ӷ���ʽ��֪��
5Ca(OH)2+5Cl2=5Ca2++2ClO-+ClO3-+7Cl-+5H2O�����������������ʵ���Ϊ5mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը������������ԭ��Ӧ����Ԫ�ػ��ϼ۴�+3�����������䱬ը��IJ����� ����Ԫ�ػ��ϼ��д���+3�ۺ�С��+3�۵Ļ����A������Ԫ�ػ��ϼ�Ϊ-1��0�ۣ������ϣ�B����Ԫ�ػ��ϼ�-1��+1�ۣ������ϣ�C����Ԫ�ػ��ϼ�Ϊ+5��+7�ۣ������ϣ�D����Ԫ�ػ��ϼ�Ϊ-1��+5�ۣ����ϣ��ʴ�ΪD��
��5�����ݻ��ϼ۱仯��ClO-��Cl-��2e-��Fe(OH)3��FeO42��3e-������ת������6��������ӷ���ʽ�ĵ����غ㡢����غ㡢ԭ���غ���з�����ƽ��д�����ӷ���ʽΪ2Fe(OH)3��3ClO-+4OH-=2FeO42-+3Cl-+5H2O��