��Ŀ����
�������������������ǿ�������������ɷ�����H1N1���С�
��1����̼������һ���ж�����;��������ϵ��̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ�
��H2O2��ʱ����Ϊ��ҵ��Һ���������������ɿ�ҵ��Һ�е��軯��(��NaCN)�������·�Ӧʵ�֣�NaCN��H2O2��H2O=A��NH3������������A�Ļ�ѧʽ______________
��ijǿ���Է�Ӧ��ϵ�У���Ӧ��������ﹲ�������ʣ�
O2��MnO4-��H2O��Mn2����H2O2��H������֪�÷�Ӧ��H2O2ֻ���������¹��̣�H2O2�� O2��
д���÷�Ӧ�����ӷ���ʽ��_______________________________________________��
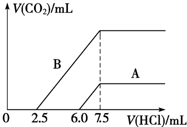
��2��ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ:aNa2CO3��bNaHCO3��2H2O��ȡm g��Ȼ������ˮ�����Һ��������Һ����μ���1 mol/L�����ᣬ��״���²�����CO2������������������֮��Ĺ�ϵijͬѧ��������ͼ��ʾ��A��B���ߣ��Իش��������⣺
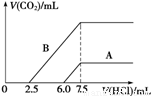
��_______������ȷ����Ȼ��Ļ�ѧʽΪ___________��
�ڼ���������CO2�������(��״��)�����ֵΪ _____________mL��
��3�� ����������ȱλ����пZnFe2Oy��������NOx��Ⱦ��ʹNOxת��ΪN2��ͬʱZnFe2Oyת��ΪZnFe2O4����2 mol ZnFe2Oy������NO2������0.5 mol N2����y��_______________��
��1����NaHCO3 ��2MnO4-��5H2O2��6H��=2Mn2����8H2O��5O2��
��2��B;�� Na2CO3��NaHCO3��2H2O�� 112 ��3��3
��������
�����������1���ٻ�ѧ��Ӧ�Ĺ��̾���ԭ��������ϵĹ��̡�����������У�Ԫ�ص����༰ԭ�Ӹ������䡣�ɵ�A��ѧʽΪ��NaHCO3���÷�Ӧ��H2O2ֻ���������¹��̣�H2O2�� O2��H2O2ʧȥ���ӣ�����ԭ��������������MnO4-����Ӧ�����ת��ΪMn2+.���ԭ���غ�͵����غ㡣�ɵ����ӷ���ʽ�ǣ�2MnO4-��5H2O2��6H��=2Mn2����8H2O��5O2������2�� ��Na2CO3�����ᷴӦ�ֲ����С����ȷ�����Na2CO3+HCl=NaCl+ NaHCO3.��Na2CO3��ȫת��ΪNaHCO3����NaHCO3+HCl=NaCl+H2O+CO2����������ֻ����Na2CO3����ǰ���������ĵ��������ʵ�����ȡ�����ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ:aNa2CO3��bNaHCO3��2H2O��������NaHCO3���ʷų��������ĵ�����Ҫ��ǰ�벿�ֶࡣ��B������ȷ��n(Na2CO3)= 1 mol/L��2. 5��10-3L=2. 5��10-3 mol.n(NaHCO3)��=1 mol/L��5��10-3L=5��10-3 mol������ԭ���������̼�����Ƶ����ʵ���Ϊ��n(NaHCO3)= 5��10-3 mol-2. 5��10-3 mol.= 2. 5��10-3 mol��n(Na2CO3):n(NaHCO3)=1:1.����ij��Ȼ��Ļ�ѧʽ�ɱ�ʾΪ: Na2CO3��NaHCO3��2H2O����n(CO2)= n(Na2CO3)+n(NaHCO3)= 5��10-3 mol.V(CO2)= n(CO2)��Vm=5��10-3 mol��22.4mol/L��103ml/L=112ml. ��3�����������غ㶨�ɿɵ�2Y+X=8 �������ã�X=2��Y=3.���ʻ�ѧʽ������
���㣺���������غ㶨�ɡ�������ԭ��Ӧ����ʽ����д��̼����������ķֲ���Ӧ��֪ʶ��

 �������������������ǿ�������������ɷ�����H1N1���У�
�������������������ǿ�������������ɷ�����H1N1���У�