��Ŀ����
��ʵ��ȷ��ij��HA��������ʡ���ͬѧ�ķ����ǣ��ף��ٳ�ȡһ��������HA���Ƴ�0.1mol?L��1����Һ100mL��
����pH��ֽ�������Һ��pH������֤��HA��������ʡ�
�ң�������֪���ʵ���Ũ�ȵ�HA��Һ�����ᣬ�ֱ�����pH��1����������Һ��100mL��
�ڷֱ�ȡ��������Һ��10mL����ˮϡ��Ϊ100mL��
�۸�ȡ��ͬ���������ϡ��Һװ�������Թ��У�ͬʱ���봿����ͬ��п�����۲췴Ӧ������֤��HA��������ʡ�
��1�������������ĵڢٲ��У���Ҫ�õ��Ķ���������_______��
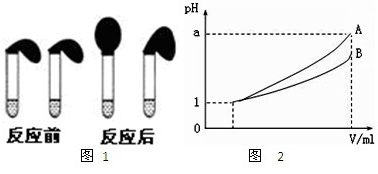
��2�������У�˵��HA��������ʵ������Dz����Һ��pH____1��ѡ���������������������
�ҷ����У�˵��HA��������ʵ�������_______����ѡ�۷֣���
A.װHCl��Һ���Թ��зų�H2�����ʿ�
B.װHA��Һ���Թ��зų�H2�����ʿ�
C.�����Թ����������������һ����
��3���������ۣ��ҷ���������ʵ��֮���Ͳ���֮��Ϊ________________��______________________��
��4�����������һ���������Ƚ������еķ�����ҩƷ����ȡ��������Ҫ������
�𣺣ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��1��100mL����ƿ
��2������B
��3������pH=1������HA��Һ����ʵ�֣�����֮�����ڼ����п�����������������ͬ
��4������NaA��Һ���ڳ����²���pH��������˵��HA������
�����������
��ʵ��������������֪ʶ�����ʵ�������һ���ܺõ���Ŀ��������������һЩ�й�ǿ��������ʵ���Ҫ֪ʶ��ͬʱ��������ͬѧ�����Ӧ����ѧ֪ʶ����ʵ������ʵ����ۺ�������
֤��HA��������ʵ�ԭ����������һ��֤��HA������ȫ���룬��Һ�д��ڵ���ƽ�⣻����֤��HA��ǿ�Ӧ���ɵ��ξ��м��ԣ���Һ�д���ˮ��ƽ�⡣�����мס�����ͬѧ���������˵�һ��ԭ����
��1�������������У����漰��Һ�����ƣ�������һ�����ʵ���Ũ�ȵ���Һ������Ķ�������������ƿ���ڻش����⣨1��ʱ��Ӧע�⣺����ƿ����һ�����ģ�����ע�����ݻ�Ϊ100mL��
��2�������е�100mL0.1mol?L��1HA��Һ����HAΪ���ᣬHA������ȫ���룬����Һ��H+��Ũ��С��0.1mol?L��1������Һ��pHӦ�ô���1��
�ҷ����У���pH=1�������HA��Һ��ϡ��10����������ǿ�ᣬ��Һ�У�H+��=0.01mol?L��1���������ˮϡ��ʱ�����������������в���HA���ӵ����H+��ʹH+�����ʵ������ӣ�����HA��Һ�У�H+�ݣ�0.01mol?L��1����ϡ�ͺ�HA��Һ��H+��Ũ�ȴ���������Һ��H+��Ũ�ȣ�H+��Ũ��Խ��Ӧ����Խ�죬���Լ�����ͬ���ȵ�п��ʱ��HA��Һ�в������������ʿ졣
��3���ҷ����У�Ҫ������һ��pH��������Һ������������Һ�д��ڵ���ƽ�⣬Ӱ�����ƽ������أ����¶ȵȣ��ܶ࣬�������Ƶ���Һ��pH���ȶ����������pH=1������HA��Һ����ʵ�֡�
п������ķ�Ӧ��Һ�������ķ�Ӧ����Ӧ���ʳ�����H+Ũ���й��⣬����ı�����Է�Ӧ���ʵ�Ӱ��Ҳ�ϴ������п�����������������ͬ�����Խ����÷�Ӧ����������������ж���Һ��H+Ũ�ȵĴ�С��˵������ǿ��
��4��Ҫ֤��ij����������ʻ��кܶ�������磺
���������������Һ�д��ڵ���ƽ���ԭ������������£�
a.�����pH��ͬ�������� HA��Һϡ����ͬ�ı�����HA��Һ��pHС��
b.�����pH��ͬ�������� HA��Һ�ֱ�ϡ�͵���ͬ��pH��HA��Һ��ˮ���ࡣ
c.��ͬpH��������HA��Һ�зֱ������Ӧ�����ι��壬HA��Һ��pH�仯��
������HA��ǿ�Ӧ���ɵ��ξ��м��ԣ���Һ�д���ˮ��ƽ���ԭ����������£�
����������NaA��Һ���ⶨ����Һ��pH����pH��������˵��HA�����ᡣ
��������Һ������ǿ���仯��������£�
a.�������ʵ���Ũ����ͬ��һԪǿ�ᣨ�����ᣩ��HA��Һ�����䵼����������HA��Һ�ĵ���������һԪǿ���������˵��HA�����ᡣ
b.���������Ʋ��ɴ���HA��ʼ��Ȼ������������ˮϡ�͵Ĺ����У������ı仯���������������ͨ���ĵ�����С����Ȼ�����ɴ�С����˵��HA�����ᡣ
���Ϸ����в�NaA��Һ��pHΪ���������ķ�����

 �ס�����λͬѧ�����ʵ��ȷ��ij��HA��������ʣ����ڵ���ƽ�⣬�Ҹı�����ƽ�ⷢ���ƶ���ʵ�鷽�����£�
�ס�����λͬѧ�����ʵ��ȷ��ij��HA��������ʣ����ڵ���ƽ�⣬�Ҹı�����ƽ�ⷢ���ƶ���ʵ�鷽�����£�