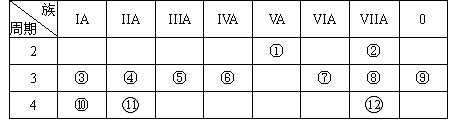
��Ŀ����
�ס��ҡ�����������Ϊԭ��������������Ķ�����Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���ĸ�һ������������������Ӳ�8�����ӡ��ס�����ɵij�������X��ʹʪ��ĺ�ɫʯ����ֽ��������ĵ�����X��Ӧ�������ҵĵ��ʣ�ͬʱ������������ˮ�������ԵĻ�����Y��Z��0.1mol/L��Y��ҺpH��1�����ĵ��ʼ������Ԫ������������ˮ�������Һ��Ӧ������LҲ����Z��ˮ��Һ��Ӧ������N�����������ɻ�����M����ش��������⣺
��1�������ӵĽṹʾ��ͼΪ ��
��2��д���ɼ�����Ԫ���γɵĻ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ������ʵĽṹʽ ��������������ڼ��������¿ɹ���ȼ�ϵ�أ��õ�طŵ�ʱ�������ķ�Ϊ ��
��3����ĵ�����X��Ӧ���ɵ�Y��Z�����ʵ���֮��Ϊ2��4����Ӧ�б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ ��
��4��д������Z��ϡ��Һ�������L��ϡ��Һ�з�����Ӧ�����ӷ���ʽ ��
��5������ͼ���M�ı�����Һ:
д���õ����з�����Ӧ���ܷ�Ӧ����ʽ ������ֵ���������Һ��μ��뵽��̪��Һ�У��۲쵽�������� ��
��1��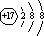
��2��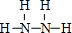 N2H4+4OH--4e-=N2��+2H2O
N2H4+4OH--4e-=N2��+2H2O
��3��2��3
��4��AlO2-+H++H2O�TAl(OH)3��
��5��2NaCl+H2O NaClO+H2�� ��Һ������ɫ
NaClO+H2�� ��Һ������ɫ
����

ij������A����TK���¾���Ļ����ṹ��Ԫ������ͼ��ʾ��T K����ת��Ϊ����ͼ��ʾ�ṹ�Ļ����ṹ��Ԫ�������־��������ڽ���Aԭ�Ӽ������ͬ ��������
��������
��1����T K���µĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ______������T K���ϵĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ___________��
��2����A�����ھ���ת��ǰ�������ṹ��Ԫ�ı߳�֮��Ϊ��TK������TK����֮�ȣ�___________��
��3������ͼ�ĵĶѻ���ʽΪ������������������ TK���¾��ⶨ��ṹ�����ʲ������±���ʾ
| ���� | ���ԭ������ | ���� | ԭ�Ӱ뾶/pm | �ܶ�/g���M-3 | ԭ�ӻ���/kJ��mol-1 |
| Na | 22.99 | s�� | 186 | 0.960 | 108.4 |
| A | 60.20 | d�� | r | 7.407 | 7735 |
��������������������������������������������������������������������������
����֪
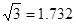 ��7.407��
��7.407�� ,1pm=10
,1pm=10 m��
m�� (14��)A��B��C��DΪԭ������������������ֵ�������Ԫ�أ��䵥�ʼ��仯��������ʻ�ṹ��Ϣ���±�������
| Ԫ�� | A | B | C | D |
| ���� �ṹ ��Ϣ | ��ҵ�ϳ��õ������AD2��ȡA�ĵ��ʡ� | B�����������������ڲ��������1�� | C������������ˮ���ᆳ�ۺϡ���ˮ��������װʳƷ������� | D��һ����̬������Կ���������ܶ�Ϊ3��������ˮ�õ���һ���ʵ���������Һ(��Ư����)������Һ���պ�������ǿ�� |
(1)��ҵ����ȡA�ĵ��ʵĻ�ѧ����ʽ�� ��
(2)��25oC��10l kPa�£���֪13.5g��B�Ĺ��嵥����D�����嵥������ȫȼ�պ�ָ���ԭ״̬������419 kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(3)Sn��Ԫ��Cͬ���壬��������Ԫ��C�Ĵ�2����Sn��ԭ������Ϊ ������SnCl2��Һʱ���������ܽ��侧�壬��Ŀ���� ��
(4)���и�ԭ�Ӷ�����8�����ȶ��ṹ�������ʽΪ ��������ˮ�����һ��ʱ����Һ������ǿ��ԭ��(�����ӷ���ʽ��ʾ) ��
(5)��A�ĵ��ʡ�B�ĵ��ʰ��õ������Ӻ����ϡNaOH��Һ�С�д��������Ӧ�ĵ缫����ʽ ��
��12�֣���ͬѧ��ͨ��ʵ��̽��ͬ����Ԫ�����ʵĵݱ���ɣ��Լ�Ӱ�컯ѧ��Ӧ���ʵ����ء�����Ƶ�ʵ�鷽�����£����������д���������������ۡ�
ʵ�����ṩ���Լ���п�顢п�ۡ����������ۡ�ͭ����NaBr��Һ��NaI��Һ�����Ƶ���ˮ��1mol/L���ᡢ3mol/L������
��1��̽��ͬ����Ԫ�����ʵĵݱ����
��д������ʵ�鱨���е�ʵ����������ӷ���ʽ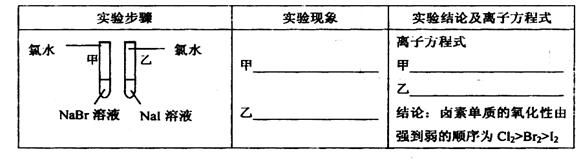
�ڡ����ۡ���ͬѧ��Ƶ�ʵ�鷽���ĺ�������___________������ţ�
A. �dz����� B. ���ֺ��� C. ��ȫ������
�ۡ���������ơ���һ�������ѡA����������������ѡB��C�������ʵ�黹��Ҫ���Լ���___________��
��2��̽��Ӱ�컯ѧ��Ӧ���ʵ�����
| ʵ�鲽�� | ʵ������ | ʵ�����ݺͽ��� |
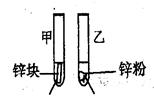 3mL1mol/L 3mL3mol/L ���� ���� ������������ͬ������������� | �����������ɣ�п������ʧ��п�����ʧ | ��Ϊ���Թ��еķ�Ӧ���죬���Է�Ӧ��Ũ��Խ��Ӧ����Խ�졣 |
��3����������ԭ���ԭ�������ʵ�飬�Ƚ�ͭ�����Ľ�����ԡ�����ʵ��װ��ͼ�������������������������д���缫��Ӧʽ��
�±����г����ֶ�����Ԫ��A��B��C��D��E����Ϣ�����ƶϺ�ش�
| Ԫ�� | �� �� �� Ϣ |
| A | Ԫ����Ҫ���ϼ�Ϊ-2��ԭ�Ӱ뾶Ϊ0.074 nm |
| B | ��������������������������֮��Ϊ4 |
| C | ԭ�Ӱ뾶Ϊ0.102 nm���䵥��Ϊ��ɫ���壬����A�ĵ�����ȼ�� |
| D | ����������ˮ�����ܰ�1�U1�������������ȵ����������� |
| E | ԭ�Ӱ뾶Ϊ0.075 nm������������ˮ����������⻯���γ�һ����X |
��1��д��CԪ�������ڱ��е�λ���� ��д��DԪ������������ˮ�������ʽ������ ��
��2��д��B������ˮ��Ӧ�����ӷ���ʽ ��
��3��Ԫ��A��D�γɵ�ij�ֻ��������Ϊ�����������������Դ��д���õ�������Ӧ����Ҫ��ѧ����ʽ ��
��4��X��ˮ��Һ������ ������ᡱ��������С����ԣ������ӷ���ʽ������ԭ�������� ��
��5����֪EԪ�ص�ij���⻯��Y��A2��Ħ��������ͬ��Y�������ɵ�ȼ�ϵ���У��������Һ��30����KOH��Һ���õ�طŵ�ʱ�����ĵ缫��ӦʽΪ ��
��6����ʹ��Y������ȼ�ϵ�ؾ���ͭ�����õ���ͭ80 gʱ��ȼ�ϵ����ת�Ƶĵ�����Ϊ NA��
Ԫ�ص�������Ԫ�����ڱ��а������г���һ���Ĺ��ɡ��±�ΪԪ�����ڱ��в���Ԫ�أ�����Ҫ��ش��������⣺
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge |
��1��������Ԫ�صĻ�̬ԭ�ӵĵ����Ų���4s�����ֻ��1�����ӵ�Ԫ���� ����Ԫ�����ƣ���
��2������Ԫ��ԭ�ӵ���Χ�����Ų����������ɽ�Ԫ�����ڱ��ֳ��������s����p����d����ds����f��������12��Ԫ�طֱ�����s����d����ds����p����������s����Ԫ���� �֣�����d����Ԫ���� �֡�
��3��ͭ���������������ṹ����ռ�������Ϊ ���ú����С��͡�
 ����ʽ�ӱ�ʾ������������
����ʽ�ӱ�ʾ������������