��Ŀ����
��10�֣������������MnC2O4��2H2O����һ�����ԳƳ������������м��ȡ������������¶ȵı仯��ϵ��ͼ��ʾ��
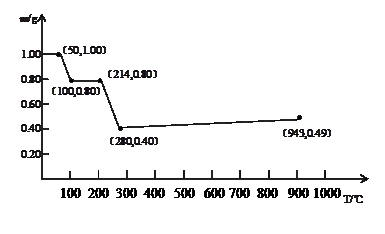
��1��214�棬ʣ�����ijɷ�Ϊ ����д��ѧʽ����ͬ����280��ʱ��ʣ�����ijɷ�Ϊ ��
��2�����¶ȳ���280��ʱ��ʣ�����������ֻ����ӣ�943����ȫ������һ�ֹ������ʣ���280��-943������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3�����������ȿ�����MnO2��MnO2��һ����Ҫ�������ܲ��ϣ�����������Һ�м���NaClO������MnO2��Cl2����д������������Һ�м���NaClOʱ������Ӧ�Ļ�ѧ����ʽ ��

��1��214�棬ʣ�����ijɷ�Ϊ ����д��ѧʽ����ͬ����280��ʱ��ʣ�����ijɷ�Ϊ ��
��2�����¶ȳ���280��ʱ��ʣ�����������ֻ����ӣ�943����ȫ������һ�ֹ������ʣ���280��-943������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��3�����������ȿ�����MnO2��MnO2��һ����Ҫ�������ܲ��ϣ�����������Һ�м���NaClO������MnO2��Cl2����д������������Һ�м���NaClOʱ������Ӧ�Ļ�ѧ����ʽ ��
��1��MnC2O4�� MnO--------6�֣�3��+3�֣�
��2��2MnO + O2 2MnO2------2��
2MnO2------2��
��3��MnSO4 + 2NaClO = MnO2 + Cl2 ��+ Na2SO4-------2��
��2��2MnO + O2
 2MnO2------2��
2MnO2------2����3��MnSO4 + 2NaClO = MnO2 + Cl2 ��+ Na2SO4-------2��
��1��1gMnC2O4��2H2O�нᾧˮ��������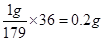 ������ͼ���֪��214�������������2hi0.2g�����Դ�ʱ������MnC2O4�����ʱ�����Ħ��������x����
������ͼ���֪��214�������������2hi0.2g�����Դ�ʱ������MnC2O4�����ʱ�����Ħ��������x����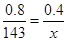 �����x��71��������MnO��
�����x��71��������MnO��
��2�������к��������������ڼ��ȵ������£��ܰ�MnO���������ʱ�����Ħ��������y����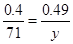 �����y��87������Ӧ���Ƕ������̣�����ʽΪ2MnO + O2
�����y��87������Ӧ���Ƕ������̣�����ʽΪ2MnO + O2 2MnO2��
2MnO2��
��3���������ƵĻ�ԭ������������1mol�������õ�1mol���ӣ���1mol��ԭ��ʧȥ2mol���ӣ����Է���ʽΪMnSO4 + 2NaClO = MnO2 + Cl2 ��+ Na2SO4��
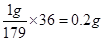 ������ͼ���֪��214�������������2hi0.2g�����Դ�ʱ������MnC2O4�����ʱ�����Ħ��������x����
������ͼ���֪��214�������������2hi0.2g�����Դ�ʱ������MnC2O4�����ʱ�����Ħ��������x����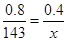 �����x��71��������MnO��
�����x��71��������MnO����2�������к��������������ڼ��ȵ������£��ܰ�MnO���������ʱ�����Ħ��������y����
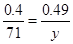 �����y��87������Ӧ���Ƕ������̣�����ʽΪ2MnO + O2
�����y��87������Ӧ���Ƕ������̣�����ʽΪ2MnO + O2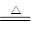 2MnO2��
2MnO2����3���������ƵĻ�ԭ������������1mol�������õ�1mol���ӣ���1mol��ԭ��ʧȥ2mol���ӣ����Է���ʽΪMnSO4 + 2NaClO = MnO2 + Cl2 ��+ Na2SO4��

��ϰ��ϵ�д�
 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
�����Ŀ