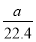��Ŀ����
���ڿ���������������ҪӦ�á�ij��ȤС����0.50mol��L-1KI��0.2��������Һ��0.20mol��L-1K2S2O8��0.10mol��L-1Na2S2O3���Լ���̽����Ӧ�����Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��֪��S2O82-+2I-�T2SO42-+I2��������I2+2S2O32-�TS4O62-+2I- ���죩��
��1����KI��Na2S2O3����۵Ļ����Һ�м���һ������K2S2O8��Һ������Һ�е�Na2S2O3�ľ�����Һ��ɫ������ɫ���Ϊ ɫ��Ϊȷ���ܹ۲쵽������S2O32����S2O82����ʼ�����ʵ���������ķ�ΧΪ��n��S2O32������n��S2O82���� ��
��2��Ϊ̽�ַ�Ӧ��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��Ƶ�ʵ�鷽�����±���
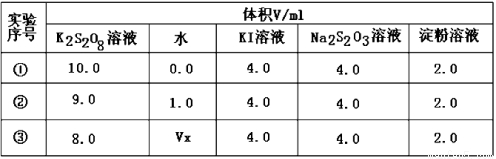
����Vx= mL����Ӧ���������� ������ţ���
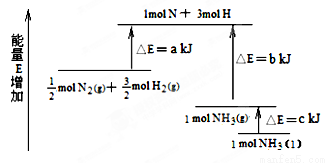
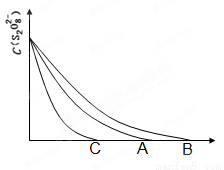
��3����֪ͼ��A����Ϊij�����£�Ũ��c��S2O82������ ��Ӧʱ��t�ı仯����ͼ��������
�����������䣬 ������B������C��������Ϊ���ͷ�Ӧ�¶ȣ� ������B������C��������Ϊ���������
��1������<2
(2)2.0����
��3��B��C
��������
�����������1������֪�ɵã���KI��Na2S2O3����۵Ļ����Һ�м���һ������K2S2O8��Һ���ȷ�S2O82-+2I-�T2SO42-+I2������������I2+2S2O32-�TS4O62-+2I- ���죩����S2O32-�ľ�����������������ʾ��ɫ�����ݷ���ʽS2O82-+2I-�T2SO42-+I2֪������1mol����Ϊ1molS2O82-������I2+2S2O32-�TS4O62-+2I- ����ʽ֪��I2��S2O32-�����ʵ����Ĺ�ϵΪ1��2����1mol����2molS2O32-��ǡ�÷�Ӧʱn��S2O32-����n��S2O82-��=2��1��Ϊȷ���ܹ۲쵽��ɫ��������ʣ�࣬n��S2O32-��Ӧ����������n��S2O32-����n��S2O82-����2��1��
��2����ʵ����̽��K2S2O8��ҺŨ�ȵı仯�Է�Ӧ���ʵ�Ӱ�죬ʵ������ʵ�������գ���Һ���һֱ��10mL,Ϊȷ����Һ������䣬����Vx=2.0mL����Ӧ����������K2S2O8��ҺŨ����������
��3���¶Ƚ��ͷ�Ӧ���ʼ�С����Ӧʱ��ӳ���ӦΪB���ߣ�ʹ�ô�������Ӧ���ʼӿ죬��Ӧʱ��̣�ӦΪC���ߡ�
���㣺�����Ӱ�췴Ӧ���ʵ����صķ����ж�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�