��Ŀ����
1840���˹����һϵ��ʵ����ʵ�ó����ɣ���ָ����������һ����Ӧ���Էֲ����У��������Ӧ�����ջ�ų��������ܺ��������Ӧһ�η���ʱ���ջ�ų���������ͬ��������18���ͷ��ֵ�һ����Ҫ���ɣ���Ϊ��˹���ɣ���֪1mol ���ʯ��ʯī�ֱ�����������ȫȼ��ʱ�ų�������Ϊ�����ʯ��395.41kJ��ʯī��393.51kJ������ʯת��ʯīʱ�����Ȼ������ȣ�______������ֵ��_______���ɴ˿������ȶ�����______����ȡ���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ������ʯ��ʯī�����ʵ���֮��Ϊ______���ú�Q�Ĵ���ʽ��ʾ����
���𰸡�����������1mol���ʯ��ʯī�ֱ�����������ȫȼ��ʱ�ų������������ø�˹�������������ʯת��ʯīʱ�������仯������Խ�͵�����Խ�ȶ�������ʮ�ֽ��淨��������ʯ��ʯī�����ʵ���֮�ȣ�
����⣺�ɸ�˹���ɿ�֪��Ҫ�õ����ʯת��Ϊʯī�������仯���ɽ���������������ȫȼ��ʱ�ų�������������ɣ�
��C�����ʯ���TC��ʯī����H=-395.41kJ/mol-��-393.51kJ/mol��=-1.90kJ/mol��
�����ʯת��Ϊʯī�ų�������˵��ʯī���������ͣ��Ƚ��ʯ�ȶ���
���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ��
��ʮ�ֽ��淨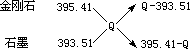 ��
��
�ɵö������ʵ�����Ϊ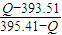 ��
��
�ʴ�Ϊ�����ȣ�1.90kJ��ʯī��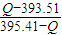 ��
��
���������⿼��ѧ�����ø�˹�������������ʯ��ʯī��ת������ȷ��Ӧ�ȡ����������������ʵ��ȶ��ԵĹ�ϵ���ɽ�𣬶��ڻ�����ȼ�ռ��ɷֵ�ȷ��ѧ��Ӧѧ������ʮ�ֽ��淨�����ٽ��
����⣺�ɸ�˹���ɿ�֪��Ҫ�õ����ʯת��Ϊʯī�������仯���ɽ���������������ȫȼ��ʱ�ų�������������ɣ�
��C�����ʯ���TC��ʯī����H=-395.41kJ/mol-��-393.51kJ/mol��=-1.90kJ/mol��
�����ʯת��Ϊʯī�ų�������˵��ʯī���������ͣ��Ƚ��ʯ�ȶ���
���ʯ��ʯī��Ͼ��干1mol ��O2����ȫȼ�գ���������ΪQkJ��
��ʮ�ֽ��淨
 ��
���ɵö������ʵ�����Ϊ
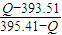 ��
���ʴ�Ϊ�����ȣ�1.90kJ��ʯī��
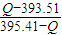 ��
�����������⿼��ѧ�����ø�˹�������������ʯ��ʯī��ת������ȷ��Ӧ�ȡ����������������ʵ��ȶ��ԵĹ�ϵ���ɽ�𣬶��ڻ�����ȼ�ռ��ɷֵ�ȷ��ѧ��Ӧѧ������ʮ�ֽ��淨�����ٽ��

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ