��Ŀ����
��1����һ�ܱ�������ʢ��aLCl2��H2�Ļ�����壬�õ����ȼ�ָ���ԭ��״̬������������ΪaL����������NaOH��Һ����ȼ�պ�����壬���������ʣ�࣮��������������֪ԭ���������Cl2��H2���ʵ���֮��һ����______��A��Cl2��H2=1 B��Cl2��H2��1 C��Cl2��H2��1 D��Cl2��H2��1
��2�������������г��ȼ�գ������ߵ������Ϊ1��2�����û������200mL 3.00mol/L��NaOH��Һ���ܶ�Ϊ1.12g/mL��ǡ����ȫ���գ�
����ԭNaOH��Һ�������������������С����ʾ������3λС����
����������Һ��Cl-��ClO-�����ʵ���֮�ͣ�
������Һ��ClO-�����ʵ�����
���𰸡���������1����������NaOH��Һ����ȼ�պ�����壬���������ʣ�࣬�����������һ������������������Դ������
��2���ٸ���m=��V������Һ������������m=CVM�������ʵ��������ٸ�������������ʽ���м��㣻
���������������ɵ��Ȼ�����������Ʒ�Ӧ�����Ȼ��ƣ��������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ�����ԭ���غ������Һ��Cl-��ClO-�����ʵ���֮�ͣ�
�۸���ʣ������������ʵ����������������ӵ����ʵ�����
����⣺��1���⣺��Cl2+H2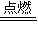 2HCl����Ӧǰ��������䣬
2HCl����Ӧǰ��������䣬
��������NaOH��Һ����ȼ�պ�����壬���������ʣ�࣬
��������Ӧ����������Ӧ��
�����������һ�����������������
��Cl2��H2��1��
��ѡC��
��2������Һ������m=��V=1.12g/mL×200mL=224g�����ʵ�����m=CVM=3.00mol/L×0.2L×40g/mol=24g����������������=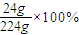 =10.7%��
=10.7%��
������������Һ������������10.7%��
���������������ɵ��Ȼ�����������Ʒ�Ӧ�����Ȼ��ƣ��������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ�2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪��n��Cl2��= n��NaOH��=
n��NaOH��= ×3.00mol/L×0.2L=0.3mol���ٸ���ԭ���غ�֪��������Һ��Cl-��ClO-�����ʵ���֮��=2 n��Cl2��=0.6mol��
×3.00mol/L×0.2L=0.3mol���ٸ���ԭ���غ�֪��������Һ��Cl-��ClO-�����ʵ���֮��=2 n��Cl2��=0.6mol��
��������Һ��Cl-��ClO-�����ʵ���֮��Ϊ0.6mol��
�۸���H2+Cl2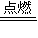 2HCl��2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪����һ����������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ��������������Ƶķ�Ӧ����һ����������ɴ������ƣ�����һ����
2HCl��2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪����һ����������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ��������������Ƶķ�Ӧ����һ����������ɴ������ƣ�����һ���� ���������ɴ������ƣ�����Һ��ClO-�����ʵ���=
���������ɴ������ƣ�����Һ��ClO-�����ʵ���= n��Cl2��=0.15mol��
n��Cl2��=0.15mol��
����Һ��ClO-�����ʵ�����0.15mol��
���������⿼�������ʵ������йؼ��㣬����ԭ���غ㡢���ʼ�ķ�Ӧ���м��㣬�Ѷ��еȣ�
��2���ٸ���m=��V������Һ������������m=CVM�������ʵ��������ٸ�������������ʽ���м��㣻
���������������ɵ��Ȼ�����������Ʒ�Ӧ�����Ȼ��ƣ��������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ�����ԭ���غ������Һ��Cl-��ClO-�����ʵ���֮�ͣ�
�۸���ʣ������������ʵ����������������ӵ����ʵ�����
����⣺��1���⣺��Cl2+H2
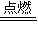 2HCl����Ӧǰ��������䣬
2HCl����Ӧǰ��������䣬��������NaOH��Һ����ȼ�պ�����壬���������ʣ�࣬
��������Ӧ����������Ӧ��
�����������һ�����������������
��Cl2��H2��1��
��ѡC��
��2������Һ������m=��V=1.12g/mL×200mL=224g�����ʵ�����m=CVM=3.00mol/L×0.2L×40g/mol=24g����������������=
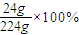 =10.7%��
=10.7%��������������Һ������������10.7%��
���������������ɵ��Ȼ�����������Ʒ�Ӧ�����Ȼ��ƣ��������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ�2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪��n��Cl2��=
 n��NaOH��=
n��NaOH��= ×3.00mol/L×0.2L=0.3mol���ٸ���ԭ���غ�֪��������Һ��Cl-��ClO-�����ʵ���֮��=2 n��Cl2��=0.6mol��
×3.00mol/L×0.2L=0.3mol���ٸ���ԭ���غ�֪��������Һ��Cl-��ClO-�����ʵ���֮��=2 n��Cl2��=0.6mol����������Һ��Cl-��ClO-�����ʵ���֮��Ϊ0.6mol��
�۸���H2+Cl2
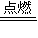 2HCl��2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪����һ����������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ��������������Ƶķ�Ӧ����һ����������ɴ������ƣ�����һ����
2HCl��2NaOH+Cl2=NaCl+NaClO+H2O��NaOH+HCl=NaCl+H2O֪����һ����������������Ʒ�Ӧ�����Ȼ��ƺʹ������ƣ��������������Ƶķ�Ӧ����һ����������ɴ������ƣ�����һ���� ���������ɴ������ƣ�����Һ��ClO-�����ʵ���=
���������ɴ������ƣ�����Һ��ClO-�����ʵ���= n��Cl2��=0.15mol��
n��Cl2��=0.15mol������Һ��ClO-�����ʵ�����0.15mol��
���������⿼�������ʵ������йؼ��㣬����ԭ���غ㡢���ʼ�ķ�Ӧ���м��㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ