��Ŀ����
ij�жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5��ֱ��С�ڵ���2.5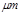 �����������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣
�����������������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣
��ش��������⣺
��1����PM2.5����������ˮ�����Ƴɴ���������
����ø������������ӵĻ�ѧ��ּ���Ũ�����±���
| ���� | H+ | K+ | Na+ | NH4+ | SO42�� | NO3�� | Cl�� |
| Ũ��/mol��L��1 | δ�ⶨ | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
���ݱ��������ж�������pH= ��
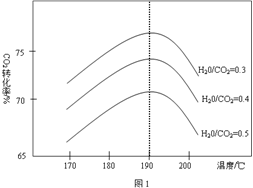
��2��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϡ�
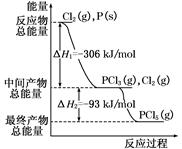
��֪��H2��g��+1/2O2��g��=H2O��g�� ��H=��241.8kJ��mol��1
C��s��+1/2O2��g��="CO" ��g�� ��H=��110.5kJ��mol��1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ�� ���� ��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ����� ��
A��Ca(OH) 2 B��Na2CO3 C��CaCl2D��NaHSO3
��3������β������NOx��CO�����ɼ�ת��
�� ��1mol������0.8molN2��0.2molO2�������еĻ�ѧ��ӦʽΪN2 (g)+O2(g)
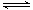 2NO(g) ��H
2NO(g) ��H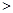 0
01300��ʱ��1mol���������ܱ������ڷ�Ӧ�ﵽƽ�⣬���NOΪ8��10��4mol��������¶��µ�ƽ�ⳣ��K= ��
���������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ����ԭ���� ��
��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NOx����Ⱦ���仯ѧ��Ӧ����ʽΪ ���������������������������������������� ��
�� ����ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO��g��=2C��s��+O2��g��
��֪�÷�Ӧ�ġ�H
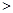 0���жϸ������ܷ�ʵ�ֲ����������ݣ� ��
0���жϸ������ܷ�ʵ�ֲ����������ݣ� ��
��1��PH=4
��2����C(s)+H2O(g)=H2(g)+CO(g) ��H=+131.3kJ/mol �� A B
��3����4��10-6���¶�Խ�ߣ���Ӧ����Խ�� ��2XCO+2NOX 2XCO2+N2
2XCO2+N2
�۲���ʵ�֣���Ϊ�÷�Ӧ�ġ�H>0,��S<0,���ԡ�G>0
����

��ϰ��ϵ�д�
�����Ŀ
��16�֣���ҵ�ϳɰ����Ʊ�����һ��������������������£�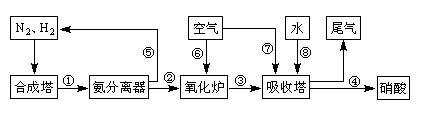
��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO(g)+H2O(g) CO2(g)+H2(g)��t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
CO2(g)+H2(g)��t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c(H2)=0.12mol��L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
��2���ϳ����з�����ӦN2(g)+3H2(g) 2NH3(g) ��H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1 300�棨�>������<����=������
2NH3(g) ��H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1 300�棨�>������<����=������
| T/�� | T1 | 300 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��3�������ڴ�����ȼ������һ�ֵ��ʺ�ˮ����ѧ�����ô�ԭ������Ƴɡ�����-������ȼ�ϵ�أ���ͨ�백���ĵ缫�� ����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ ��
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4(g)+4NO2(g)��4NO(g)+CO2(g)+2H2O(g) ��H= ��574kJ��mol��1
CH4(g)+4NO(g)��2N2(g)+CO2(g)+2H2O(g) ��H= ��1160kJ��mol��1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ ��
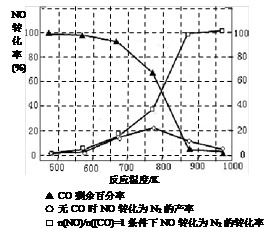
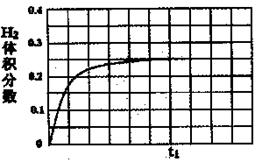
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�����ͼ����ͼ����������ʹ��CO���¶ȳ���775K������NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ ����n(NO)/n(CO)=1�������£�Ӧ���Ƶ�����¶��� ���ҡ�
 H
H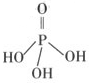
 ���ڴ����д������ƽ��Ӧʽ��a����
���ڴ����д������ƽ��Ӧʽ��a����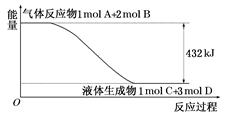
 2CO2(g)+N2(g)
2CO2(g)+N2(g)
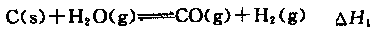


 CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� ��
CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� �� ��H����88.6 kJ��mol��1
��H����88.6 kJ��mol��1 O2(g)=CO2(g)��2H2(g) ��H����a kJ��mol��1����a 238.6(�����������������)��
O2(g)=CO2(g)��2H2(g) ��H����a kJ��mol��1����a 238.6(�����������������)�� NH2CO2NH4(s) ��H1="a" kJ��mol��1
NH2CO2NH4(s) ��H1="a" kJ��mol��1