��Ŀ����
�����ҹ�����ӵ�����ʽϿ��������ƣ�����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
��1��������ȼ������ʱ����Ӧ��N2��g��+O2��g��?2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2L���ܱ������г���10mol N2��5mol O2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��
K=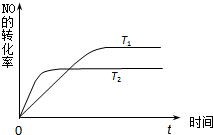
��2��һ������NO�����ֽ�Ĺ����У�NO��ת������ʱ��仯��������ͼ��ʾ������֪��T1��T2��
�ٷ�Ӧ 2NO��g��?N2��g��+O2��g��Ϊ������ȡ����ȡ���
��һ���¶��£��ܹ�˵����Ӧ 2NO��g��?N2��g��+O2��g�� �Ѵﵽƽ����ǣ�����ţ�
a�������ڵ�ѹǿ�������仯 b��NO�ֽ�����ʺ�NO���ɵ��������
c��NO��N2��O2��Ũ�ȱ��ֲ��� d����λʱ���ڷֽ�4mol NO��ͬʱ����2mol N2
��3���ٵ�����������ϡ��ȼ��ʱ��β���е���Ҫ��Ⱦ��ΪNOx������CxHy����������ԭNOx���������������Ⱦ��
��֪��CH4��g��+4NO2��g��=�T4NO��g��+CO2��g��+2H2O��g����H1=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H2
CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H3=-867kJ?mol-1 ��H2=
��ʹ�ô������Խ�����β������Ҫ�к��ɷ�һ����̼��CO���͵������NOx��ת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ
��1��������ȼ������ʱ����Ӧ��N2��g��+O2��g��?2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2L���ܱ������г���10mol N2��5mol O2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��
K=
0.11
0.11
��������������С�������λ���֣�
��2��һ������NO�����ֽ�Ĺ����У�NO��ת������ʱ��仯��������ͼ��ʾ������֪��T1��T2��
�ٷ�Ӧ 2NO��g��?N2��g��+O2��g��Ϊ������ȡ����ȡ���
����
����
��Ӧ����һ���¶��£��ܹ�˵����Ӧ 2NO��g��?N2��g��+O2��g�� �Ѵﵽƽ����ǣ�����ţ�
bc
bc
��a�������ڵ�ѹǿ�������仯 b��NO�ֽ�����ʺ�NO���ɵ��������
c��NO��N2��O2��Ũ�ȱ��ֲ��� d����λʱ���ڷֽ�4mol NO��ͬʱ����2mol N2
��3���ٵ�����������ϡ��ȼ��ʱ��β���е���Ҫ��Ⱦ��ΪNOx������CxHy����������ԭNOx���������������Ⱦ��
��֪��CH4��g��+4NO2��g��=�T4NO��g��+CO2��g��+2H2O��g����H1=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H2
CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H3=-867kJ?mol-1 ��H2=
-1160 kJ?mol-1
-1160 kJ?mol-1
����ʹ�ô������Խ�����β������Ҫ�к��ɷ�һ����̼��CO���͵������NOx��ת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ
2x CO+2NOx
2x CO2+N2
| ||
| ���� |
2x CO+2NOx
2x CO2+N2
��
| ||
| ���� |
��������1����Ϸ���ʽN2��g��+O2��g��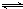 2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻
2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻
��2���ٸ���ͼ���жϣ�b�����ȵ���ƽ�⣬��Ӧ���ʴ��¶Ƚϸߣ����¶����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ���������ƶ�������Ӧ���ȣ�
�ڿ��淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ�ͬ�����ʣ������淴Ӧ����֮�ȵ���ϵ��֮�ȣ���ͬ���ʣ���ƽ��ʱ�������ʵ����ʵ�����Ũ�ȵȲ��ٷ����仯���ɴ�������һЩ���������䣬�Դ˷�����
��3���ٸ��ݸ�˹���������
�ڸ��ݻ�ѧ����ʽ����д������д����ʽ��
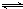 2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻
2NO��g��������ƽ������������������ʵ����ʵ�����������ƽ�ⳣ����ʽ���㣻��2���ٸ���ͼ���жϣ�b�����ȵ���ƽ�⣬��Ӧ���ʴ��¶Ƚϸߣ����¶����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ���������ƶ�������Ӧ���ȣ�
�ڿ��淴Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ�ͬ�����ʣ������淴Ӧ����֮�ȵ���ϵ��֮�ȣ���ͬ���ʣ���ƽ��ʱ�������ʵ����ʵ�����Ũ�ȵȲ��ٷ����仯���ɴ�������һЩ���������䣬�Դ˷�����
��3���ٸ��ݸ�˹���������
�ڸ��ݻ�ѧ����ʽ����д������д����ʽ��
����⣺��1��N2��g��+O2��g��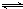 2NO��g����
2NO��g����
��ʼ��mol�� 10 5 0
��Ӧ��mol�� 1 1 2
ƽ�⣨mol�� 9 4 2
ƽ��ʱŨ��Ϊ��c�� N2��=
=
=4.5mol/L��c��O2��=2mol/L��c��NO��=1mol/L��K=
=0.11���ʴ�Ϊ��0.11��
��2�����¶����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ���������ƶ�������Ӧ���ȣ��ʴ�Ϊ������
��a���÷�Ӧ�Ƿ�Ӧ�����������ķ�Ӧ���������۷�Ӧ�Ƿ�ﵽƽ��״̬����ϵ��ѹǿʼ�ղ��䣬��a����
b��NO�����淴Ӧ������ȣ����Դﵽƽ��״̬����b��ȷ��
c��NO��N2��O2��Ũ�ȱ��ֲ��䣬�ﵽƽ��״̬����c��ȷ��
d����λʱ��������4molNO��ͬʱ����2molN2����������Ӧ���ʣ�����˵����Ӧ�ﵽƽ��״̬����d����
�ʴ�Ϊ��bc��
��3����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ?mol-1 ��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 ��
���ݸ�˹���ɵã�2CH4��g��+4NO2��g��=2CO2��g��+4H2O��g��+2N2��g����H1+��H2
1mol CH4��ԭNO2��N2�����������зų�������Ϊ867kJ�����ԡ�H1+��H2=-1734kJ?mol-1�����H2=-1734kJ?mol-1+574kJ?mol-1=-1160 kJ?mol-1���ʴ�Ϊ��-1160kJ?mol-1��
�ڵ����������NOX��ʾ����CO���Ӧ������N2��CO2���仯ѧ����ʽΪ��2NOx+2xCO�T2xCO2+N2��
�ʴ�Ϊ��2NOx+2xCO�T2xCO2+N2
 2NO��g����
2NO��g������ʼ��mol�� 10 5 0
��Ӧ��mol�� 1 1 2
ƽ�⣨mol�� 9 4 2
ƽ��ʱŨ��Ϊ��c�� N2��=
| n |
| V |
| 9mol |
| 2L |
| 12 |
| 4.5��2 |
��2�����¶����ߣ�NO��ת���ʼ�С��˵�������¶�ƽ���������ƶ�������Ӧ���ȣ��ʴ�Ϊ������
��a���÷�Ӧ�Ƿ�Ӧ�����������ķ�Ӧ���������۷�Ӧ�Ƿ�ﵽƽ��״̬����ϵ��ѹǿʼ�ղ��䣬��a����
b��NO�����淴Ӧ������ȣ����Դﵽƽ��״̬����b��ȷ��
c��NO��N2��O2��Ũ�ȱ��ֲ��䣬�ﵽƽ��״̬����c��ȷ��
d����λʱ��������4molNO��ͬʱ����2molN2����������Ӧ���ʣ�����˵����Ӧ�ﵽƽ��״̬����d����
�ʴ�Ϊ��bc��
��3����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ?mol-1 ��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 ��
���ݸ�˹���ɵã�2CH4��g��+4NO2��g��=2CO2��g��+4H2O��g��+2N2��g����H1+��H2
1mol CH4��ԭNO2��N2�����������зų�������Ϊ867kJ�����ԡ�H1+��H2=-1734kJ?mol-1�����H2=-1734kJ?mol-1+574kJ?mol-1=-1160 kJ?mol-1���ʴ�Ϊ��-1160kJ?mol-1��
�ڵ����������NOX��ʾ����CO���Ӧ������N2��CO2���仯ѧ����ʽΪ��2NOx+2xCO�T2xCO2+N2��
�ʴ�Ϊ��2NOx+2xCO�T2xCO2+N2
���������⿼�黯ѧƽ�⼰��ؼ��㣬֪ʶ��϶࣬�ѶȲ���

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
�����Ŀ
 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol
N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol
N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��