��Ŀ����
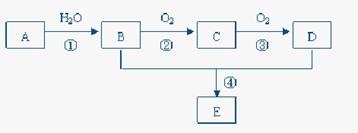
��16�֣� I��ʵ�����г���BaSO4�������ⶨBaCl2��nH2O�е�Ba�ĺ�����Ҫ�������£�
�� ���մ����������أ��Ƶ�����������
�� ��ȡBaCl2��nH2O�����ձ����ܽ⣬�����ᴦ��
�� ��һ��Ũ�ȵĹ���������뱵���еõ���ɫ����
�� ���ã�
�� ����������ֽ���ڴ������У����ƾ���Ƽ���̼��������800-850�����������أ�����
�� ����
��ش��������⣺
��1��ʡ�Եڢٲ��С����մ����������ء����ܵ��¼�����_ ______���ƫ�ߡ��������䡱��ƫ�͡�����
______���ƫ�ߡ��������䡱��ƫ�͡�����
��2���ڢܲ����ú�IJ����� ��
��3���ڢݲ������¶Ȳ�����900�棬����Ϊ_______________________��
II��ijͬѧ�ð�ˮ����һ������SO2�����պ���Һ�п��ܺ���OH-��SO32-��SO42-�� HSO3- ���������е������֡�
��4��д����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽ��
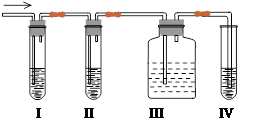
��5����֪����������һ�������ˮ������ѡ����������Լ����ձ����Թܡ�����������ͷ�ιܣ� 2 mol/L���ᡢ2 mol/L���ᡢ1 mol/L�Ȼ�����Һ��l mol/L����������Һ��Ʒ����Һ������ˮ�������ʵ��̽�����պ���Һ���Ƿ����SO32-��HSO3-����ʵ�������Ԥ�ڵ�ʵ������ͽ��������±��С�
| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ�1�У��μӹ���lmol/L�Ȼ�����Һ�� | �������ֻ��ǣ�����Һ�в�����SO32-�� �����ֻ��ǣ�����Һ�п��ܺ���SO32-�� |
| ����2�������ֻ��ǣ�����һ��ʱ����ϲ���Һ�����Թ�2�С����Թ�1�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ���ټ��� �� | |
| ����3�� | |
��16�֣�
��1��ƫ�� ��2�֣� ��2�����ˡ�ϴ�ӳ�����2�֣�
��3�����ᱵ�ᱻ̼��ԭ���������ᱵ�ᷢ���ֽ⡱����2�֣�
��4��NH3��H2O + SO2 = NH4+ + HSO3- ��2�֣�
��5��ʵ����� Ԥ����������� ����2�� 2mol/L���ᡣ��1�֣� ���������������壬��֤����Һ�д���SO32-��
�������壬����SO32-����2�֣�����3�����Թ�2�м������2mol/L���ᣬ�ٵ���2��Ʒ�졣
�����Թ�2�м������lmol/L����������Һ����3�֣���ɫ��ȥ�������HSO3-����ɫ����ȥ������HSO3-��
���ֻ��ǣ������HSO3-�������ֻ��ǣ�����HSO3-����2�֣�
����