��Ŀ����
��2013?��ͨһģ����ԭ����ԭ����������������绯ѧ���շ��Ǽ��ٵ��������ŷŵ���Ч��ʩ��
��1������̿�ۿ��Խ��������ﻹԭ��
��֪��N2��g��+O2��g���T2NO��g����H=+180.6kJ?mol-1
C��s��+O2��g���TCO2��g����H=-393.5kJ?mol-1
��Ӧ��C��s��+2NO��g���TCO2��g��+N2��g����H=
��2��TiO2�������������»�ʹ�����е�ijЩ���Ӳ������Ի���OH��OH�ܽ�NO��NO2��������ͼ����ʾ��OH��NO2�ķ�ӦΪNO2+OH�THNO3��д��OH��NO��Ӧ�Ļ�ѧ����ʽ��
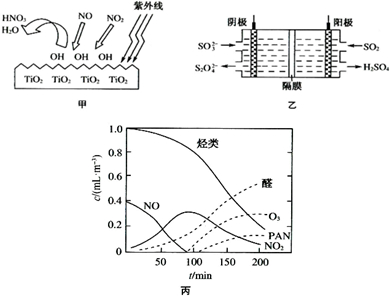
��3��ͼ����ʾ��װ�������պ�ת��NO2��NO��SO2��
���������ĵ缫��ӦʽΪ
�������ų�����Һ�к�S2
������������NOx���壬���ɵ�S
������������������S2
����NO2�����ӷ���ʽ��ƽ�����ڷ�����������Ӧ���ʣ�
+
+
����֪�������ɵ�����Һÿ���ձ�״����7.84L�����壬��������������������Ϊ49%������100g��������������NO2��NO�����ʵ���֮��Ϊ
��4��O3��ȩ�ࡢPAN��������������������Ⱦ������Ϳ��������γɵ�������Ϊ�⻯ѧ������ij�о���ѧϰС��Ϊģ��⻯ѧ�������γɣ�������������װ���ܱ������ڵı���Ⱦ������Ʒ���������ʵ�Ũ����ʱ��ı仯��ͼ����ʾ��������ݹ⻯ѧ�������γ�ԭ�����Լ��ٹ⻯ѧ�����ķ������һ���������飺
��1������̿�ۿ��Խ��������ﻹԭ��
��֪��N2��g��+O2��g���T2NO��g����H=+180.6kJ?mol-1
C��s��+O2��g���TCO2��g����H=-393.5kJ?mol-1
��Ӧ��C��s��+2NO��g���TCO2��g��+N2��g����H=
-574.1
-574.1
kJ?mol-1����2��TiO2�������������»�ʹ�����е�ijЩ���Ӳ������Ի���OH��OH�ܽ�NO��NO2��������ͼ����ʾ��OH��NO2�ķ�ӦΪNO2+OH�THNO3��д��OH��NO��Ӧ�Ļ�ѧ����ʽ��
3OH+NO�THNO3+H2O
3OH+NO�THNO3+H2O
��
��3��ͼ����ʾ��װ�������պ�ת��NO2��NO��SO2��
���������ĵ缫��ӦʽΪ
SO2-2e-+2H2O�TSO42-+4H+
SO2-2e-+2H2O�TSO42-+4H+
���������ų�����Һ�к�S2
| O | 2- 4 |
| O | 2- 3 |
| O | 2- 4 |
4
4
S2| O | 2- 4 |
2
2
NO2+8
8
OH-�T8
8
S| O | 2- 3 |
1
1
N2+4H2O
4H2O
����֪�������ɵ�����Һÿ���ձ�״����7.84L�����壬��������������������Ϊ49%������100g��������������NO2��NO�����ʵ���֮��Ϊ
3��4
3��4
����4��O3��ȩ�ࡢPAN��������������������Ⱦ������Ϳ��������γɵ�������Ϊ�⻯ѧ������ij�о���ѧϰС��Ϊģ��⻯ѧ�������γɣ�������������װ���ܱ������ڵı���Ⱦ������Ʒ���������ʵ�Ũ����ʱ��ı仯��ͼ����ʾ��������ݹ⻯ѧ�������γ�ԭ�����Լ��ٹ⻯ѧ�����ķ������һ���������飺
���ٻ������к�����β�����ŷ�
���ٻ������к�����β�����ŷ�
����������1�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��2������ͼ���֪NO��NO2����OH����ΪHNO3������ԭ���غ�õ���
��3�������ݵ缫ԭ���ͷ�Ӧ�����е����ӱ仯д���缫��Ӧ��
������������ԭ��Ӧ�����غ��Ԫ��ԭ���غ���ƽд�����ӷ���ʽ��
����������������0.5molH2SO4ת�Ƶ���1mol����������0.35molһ�������Ͷ��������Ļ������ת�Ƶ���1mol�����ݵ����غ����õ���
��4������ͼ�������֪NO��NO2������������������������O3��ȩ��PAN����Ⱦ��Ӷ��γɹ⻯ѧ������������β�����ŷŵ���������������Ҫ��Դ��
��2������ͼ���֪NO��NO2����OH����ΪHNO3������ԭ���غ�õ���
��3�������ݵ缫ԭ���ͷ�Ӧ�����е����ӱ仯д���缫��Ӧ��
������������ԭ��Ӧ�����غ��Ԫ��ԭ���غ���ƽд�����ӷ���ʽ��
����������������0.5molH2SO4ת�Ƶ���1mol����������0.35molһ�������Ͷ��������Ļ������ת�Ƶ���1mol�����ݵ����غ����õ���
��4������ͼ�������֪NO��NO2������������������������O3��ȩ��PAN����Ⱦ��Ӷ��γɹ⻯ѧ������������β�����ŷŵ���������������Ҫ��Դ��
����⣺��1����N2��g��+O2��g���T2NO��g����H=+180.6kJ?mol-1
��C��s��+O2��g���TCO2��g����H=-393.5kJ?mol-1
�ɸ�˹���ɢ�-�ٵ�C��s��+2NO��g���TCO2��g��+N2��g����H=-393.5kJ/mol-180.6KJ/mol=-574.1KJ/mol���ʴ�Ϊ��-574.1��
��2����ͼ���֪NO��NO2����OH����ΪHNO3������ԭ���غ�õ���NO+3OH�THNO3+H2O��
�ʴ�Ϊ��3OH+NO�THNO3+H2O��
��3��������ͼʾ��֪��������������������Ϊ�������������������ӦSO2-2e-+2H2O�TSO42-+4H+��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
�����ݵ����غ�͵���غ���ƽ������S2O42-����Ԫ��Ϊ+3�ۣ������������Ԫ��Ϊ+4�ۣ�����S2O42-�����������������еĵ�Ԫ�ػ��ϼ�Ϊ+4�ۣ�����Ϊ0�ۣ�������������ԭ�������ӷ���ʽΪ4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O��
�ʴ�Ϊ��4��2��8��8��1��4��
����������������0.5molH2SO4ת�Ƶ���1mol����������0.35molһ�������Ͷ��������Ļ������ת�Ƶ���1mol�����ݵ����غ�n��NO��+n��NO2��=0.35��2n��NO��+4n��NO2��=1��
�õ�n��NO��=0.2mol��n��NO2��=0.15mol��
����n��NO2����n��NO��=0.15��0.2=3��4��
�ʴ�Ϊ��3��4��
��4������ͼ�������֪NO��NO2������������������������O3��ȩ��PAN����Ⱦ��Ӷ��γɹ⻯ѧ������������β�����ŷŵ���������������Ҫ��Դ���ʼ�������β�����Լ��ٹ⻯ѧ�����ķ�����
�ʴ�Ϊ�����ٻ������к�����β�����ŷţ�
��C��s��+O2��g���TCO2��g����H=-393.5kJ?mol-1
�ɸ�˹���ɢ�-�ٵ�C��s��+2NO��g���TCO2��g��+N2��g����H=-393.5kJ/mol-180.6KJ/mol=-574.1KJ/mol���ʴ�Ϊ��-574.1��
��2����ͼ���֪NO��NO2����OH����ΪHNO3������ԭ���غ�õ���NO+3OH�THNO3+H2O��
�ʴ�Ϊ��3OH+NO�THNO3+H2O��
��3��������ͼʾ��֪��������������������Ϊ�������������������ӦSO2-2e-+2H2O�TSO42-+4H+��
�ʴ�Ϊ��SO2-2e-+2H2O�TSO42-+4H+��
�����ݵ����غ�͵���غ���ƽ������S2O42-����Ԫ��Ϊ+3�ۣ������������Ԫ��Ϊ+4�ۣ�����S2O42-�����������������еĵ�Ԫ�ػ��ϼ�Ϊ+4�ۣ�����Ϊ0�ۣ�������������ԭ�������ӷ���ʽΪ4S2O42-+2NO2+8OH-�T8SO42-+N2+4H2O��
�ʴ�Ϊ��4��2��8��8��1��4��
����������������0.5molH2SO4ת�Ƶ���1mol����������0.35molһ�������Ͷ��������Ļ������ת�Ƶ���1mol�����ݵ����غ�n��NO��+n��NO2��=0.35��2n��NO��+4n��NO2��=1��
�õ�n��NO��=0.2mol��n��NO2��=0.15mol��
����n��NO2����n��NO��=0.15��0.2=3��4��
�ʴ�Ϊ��3��4��
��4������ͼ�������֪NO��NO2������������������������O3��ȩ��PAN����Ⱦ��Ӷ��γɹ⻯ѧ������������β�����ŷŵ���������������Ҫ��Դ���ʼ�������β�����Լ��ٹ⻯ѧ�����ķ�����
�ʴ�Ϊ�����ٻ������к�����β�����ŷţ�
���������⿼�����Ȼ�ѧ����ʽ��˹���ɼ���Ӧ�ã�����ԭ��Ӧ�ã�������ԭ��Ӧ�����غ�ļ��㣬�������������Ϣ����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ
