��Ŀ����
��2013?Ϋ��ģ�⣩[��ѧ--�л���ѧ����]
��ͼ����AΪ��Ҫ��ʼԭ�ϵ�����M�߷��ӻ�����N�ķ�Ӧ���̣����ֲ��P��Ӧ��������ȥ����

��1��д��C�к��������ŵ����ƣ�
��2����֪D����֧����д��D��ͬ���ͬ���칹��ṹ��ʽ��
��3������M�Ļ�ѧ����ʽΪ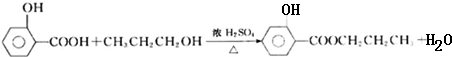
 ����Ӧ����Ϊ
����Ӧ����Ϊ
��4��д����A���ɸ߾���N�Ļ�ѧ����ʽ��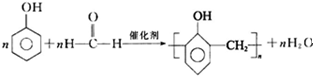
 ��
��
��ͼ����AΪ��Ҫ��ʼԭ�ϵ�����M�߷��ӻ�����N�ķ�Ӧ���̣����ֲ��P��Ӧ��������ȥ����

��1��д��C�к��������ŵ����ƣ�
���ǻ�
���ǻ�
���Ȼ�
�Ȼ�
����2����֪D����֧����д��D��ͬ���ͬ���칹��ṹ��ʽ��
CH3CHOHCH3
CH3CHOHCH3
����3������M�Ļ�ѧ����ʽΪ


������Ӧ��ȡ����Ӧ
������Ӧ��ȡ����Ӧ
����4��д����A���ɸ߾���N�Ļ�ѧ����ʽ��


������A�ͼ�ȩ������Ӧ����N��ȩ��֬����A�DZ��ӣ��ҽṹ��ʽΪ��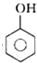 ����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C
����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C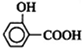 ����B�Ľṹ��ʽΪ��
����B�Ľṹ��ʽΪ��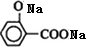 ��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ��
��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ�� ���ٸ������ʵ��������������
���ٸ������ʵ��������������
 ����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C
����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C ����B�Ľṹ��ʽΪ��
����B�Ľṹ��ʽΪ��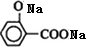 ��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ��
��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ�� ���ٸ������ʵ��������������
���ٸ������ʵ������������������⣺A�ͼ�ȩ������Ӧ����N��ȩ��֬����A�DZ��ӣ��ҽṹ��ʽΪ�� ����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C
����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C ����B�Ľṹ��ʽΪ��
����B�Ľṹ��ʽΪ��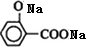 ��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ��
��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ�� ��
��
��1��C�Ľṹ��ʽΪ�� ��C�к��еĹ������Ƿ��ǻ����Ȼ����ʴ�Ϊ�����ǻ����Ȼ���
��C�к��еĹ������Ƿ��ǻ����Ȼ����ʴ�Ϊ�����ǻ����Ȼ���
��2��D��ͬ���ͬ���칹��ṹ��ʽΪCH3CHOHCH3���ʴ�Ϊ��CH3CHOHCH3��
��3������M�ķ�Ӧ����ʽΪ�� ���÷�Ӧ����������Ӧ��ȡ����Ӧ��
���÷�Ӧ����������Ӧ��ȡ����Ӧ��
�ʴ�Ϊ�� ��������Ӧ��ȡ����Ӧ��
��������Ӧ��ȡ����Ӧ��
��4�����Ӻͼ�ȩ�������۷�Ӧ���ɷ�ȩ��֬����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
 ����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C
����һ�������£����Ӻ�̼���Ʒ�Ӧ����B��B���ᷴӦ����C ����B�Ľṹ��ʽΪ��
����B�Ľṹ��ʽΪ��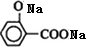 ��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ��
��C��Ũ���ᡢ���������º�D��Ӧ����M�����M�ķ���ʽ֪��D�Ǵ������D����֧������D�Ľṹ��ʽΪ��CH3CH2CH2OH��M�Ľṹ��ʽΪ�� ��
����1��C�Ľṹ��ʽΪ��
 ��C�к��еĹ������Ƿ��ǻ����Ȼ����ʴ�Ϊ�����ǻ����Ȼ���
��C�к��еĹ������Ƿ��ǻ����Ȼ����ʴ�Ϊ�����ǻ����Ȼ�����2��D��ͬ���ͬ���칹��ṹ��ʽΪCH3CHOHCH3���ʴ�Ϊ��CH3CHOHCH3��
��3������M�ķ�Ӧ����ʽΪ��
 ���÷�Ӧ����������Ӧ��ȡ����Ӧ��
���÷�Ӧ����������Ӧ��ȡ����Ӧ���ʴ�Ϊ��
 ��������Ӧ��ȡ����Ӧ��
��������Ӧ��ȡ����Ӧ����4�����Ӻͼ�ȩ�������۷�Ӧ���ɷ�ȩ��֬����Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ����չ����ŵ������Լ������ŵ�ת��Ϊ������Ĺؼ����״���Ϊͬ���칹����жϣ�ע����������Ϣ��

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 ��2013?Ϋ��ģ�⣩��ͼ������̼�����ָ������Ϣʱ����������Ҫ���٣��Ӷ�����̼�ر��Ƕ�����̼���ŷţ�������Ϊ�����ڵ�̼������ǣ�������
��2013?Ϋ��ģ�⣩��ͼ������̼�����ָ������Ϣʱ����������Ҫ���٣��Ӷ�����̼�ر��Ƕ�����̼���ŷţ�������Ϊ�����ڵ�̼������ǣ������� ��2013?Ϋ��ģ�⣩��ͼ���о�̼�������������������ʶԻ��������ͻ�����������Ҫ���壮
��2013?Ϋ��ģ�⣩��ͼ���о�̼�������������������ʶԻ��������ͻ�����������Ҫ���壮