��Ŀ����
 ��֪�����ʾ���ݣ�
��֪�����ʾ���ݣ�| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g?cm-3�� |
| �Ҵ� | -117.3 | 78.5 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | - | 338.0 | 1.84 |
����������ƿ�а������2��3��3����Ũ���ᡢ�Ҵ�������Ļ��Һ80mL��
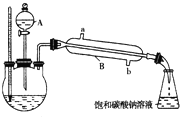
�ڰ���ͼ��ʾ���Ӻ�װ�ã�װ�����������ã������Լ��г�װ��ʡ�ԣ�����С����ȼ���װ�л��Һ��������ƿ5��10min��
�۱��ȣ��ߴ�����A��������ƿ�еμ��Ҵ���
�ܴ���ƿ�ռ���һ���������ֹͣ���ȣ���������ƿ��Ȼ���ô��ֲ㣮
�ݷ�������������㣬ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1������Aʹ��ǰ������еIJ�����
��2������B��������
��3���Ҵ������ᷴӦ�Ļ�ѧ����ʽ��
��4��ʵ����ɺ���ƿ��Һ���������IJ�����ˮ����״Һ�壬��������״Һ�������Ҫ�õ�����Ҫ����������
��5����30g������46g�Ҵ���Ӧ�����ʵ�ʲ��������۲�����67%����ʵ�ʵõ�����������������
A��44g B�� 29.3g C��74.8g D��88g��
��������1����Һ©��ʹ��ǰ��Ҫ�����Ƿ�©Һ��
��2������BΪ�����ܣ���ˮΪ�¿ڽ��Ͽڳ���
��3��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��4�����뻥�����ܵ�Һ��ͨ���÷�Һ�ķ����������������ܶȱ�ˮ��С��
��5������������Ҵ���������ϵ�жϷ�Ӧ�Ĺ������⣬��Ϸ���ʽ���㣻
��2������BΪ�����ܣ���ˮΪ�¿ڽ��Ͽڳ���
��3��������Ҵ���Ũ���������·���������Ӧ����������������ˮ��
��4�����뻥�����ܵ�Һ��ͨ���÷�Һ�ķ����������������ܶȱ�ˮ��С��
��5������������Ҵ���������ϵ�жϷ�Ӧ�Ĺ������⣬��Ϸ���ʽ���㣻
����⣺��1����Һ©��ʹ��ǰ��Ҫ�����Ƿ�©Һ���ʴ�ΪC��
��2������BΪ�����ܣ�Ϊ�˴ﵽ���õ�����Ч������ˮΪ�¿ڽ��Ͽڳ���
�ʴ�Ϊ�������ܣ�b��
��3���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬
��Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH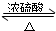 CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+HOCH2CH3 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4�����뻥�����ܵ�Һ��ͨ���÷�Һ�ķ������õ�����Ҫ����Ϊ��Һ©���������������ܶȱ�ˮ��С���������������ϲ㣬�ӷ�Һ©�����Ͽڵ�����
�ʴ�Ϊ����Һ©�����Ͽڵ�����
��5��30g��������ʵ���Ϊ
=0.5mol��46g�Ҵ������ʵ���Ϊ
=1mol��
��Ӧ�ķ���ʽΪ��CH3COOH+HOCH2CH3 CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ���������
CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ���������
CH3COOH+HOCH2CH3 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O
1mol 1mol 1mol
0.5mol��67% n
���n=0.5mol��67%=0.335mol����������������m��CH3COOCH2CH3��=0.335mol��88g/mol=29.5g��
�ʴ�Ϊ��A��
��2������BΪ�����ܣ�Ϊ�˴ﵽ���õ�����Ч������ˮΪ�¿ڽ��Ͽڳ���
�ʴ�Ϊ�������ܣ�b��
��3���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬
��Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH
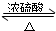 CH3COOC2H5+H2O��
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+HOCH2CH3
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����4�����뻥�����ܵ�Һ��ͨ���÷�Һ�ķ������õ�����Ҫ����Ϊ��Һ©���������������ܶȱ�ˮ��С���������������ϲ㣬�ӷ�Һ©�����Ͽڵ�����
�ʴ�Ϊ����Һ©�����Ͽڵ�����
��5��30g��������ʵ���Ϊ
| 30g |
| 60g/mol |
| 46g |
| 46g/mol |
��Ӧ�ķ���ʽΪ��CH3COOH+HOCH2CH3
 CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ���������
CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ���������CH3COOH+HOCH2CH3
 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O1mol 1mol 1mol
0.5mol��67% n
���n=0.5mol��67%=0.335mol����������������m��CH3COOCH2CH3��=0.335mol��88g/mol=29.5g��
�ʴ�Ϊ��A��
���������⿼�������������Ʊ�����Ŀ�Ѷ��еȣ�ע��ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ���������������ѧ����������������������������

��ϰ��ϵ�д�
�����Ŀ
��֪����Ԫ�ص�ԭ�Ӱ뾶�����ʾ��
�����������ݣ���ԭ�ӵİ뾶�����ǣ�������
| ԭ�� | N | S | O | Si |
| r/10-10m | 0.75 | 1.02 | 0.74 | 1.17 |
| A��1.10��10-10 m |
| B��0.80��10-10 m |
| C��1.20��0-10 m |
| D��0.70��10-10 m |