��Ŀ����
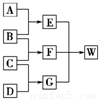
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
��1����֪EΪֱ���͵ķǼ��Է��ӣ���E�ĽṹʽΪ______��D���ʵĵ���ʽΪ______��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3����G���ӵĿռ乹��Ϊ______��
��2��C+DG�Ļ�ѧ����ʽΪ______��
��3����֪������Ԫ��X��B�е�Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��Һ��A��Ӧ�Ļ�ѧ����ʽΪ______��
��4��20��ʱ��G��Eͨ�뱥��ʳ��ˮ�У��о���������д���÷�Ӧ�Ļ�ѧ����ʽ______��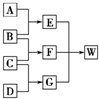
��1����֪EΪֱ���͵ķǼ��Է��ӣ���E�ĽṹʽΪ______��D���ʵĵ���ʽΪ______��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3����G���ӵĿռ乹��Ϊ______��
��2��C+DG�Ļ�ѧ����ʽΪ______��
��3����֪������Ԫ��X��B�е�Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��Һ��A��Ӧ�Ļ�ѧ����ʽΪ______��
��4��20��ʱ��G��Eͨ�뱥��ʳ��ˮ�У��о���������д���÷�Ӧ�Ļ�ѧ����ʽ______��

������������Ԫ����ɷǽ�����̬�������������������������������������ɣ�1������֪��EΪֱ���ͷǼ��Է��ӣ���ϵCO2��C2H2����G������ԭ�Ӹ�����֪��GΪ�����ٸ��ݿ�ͼ��֪��AΪ���嵥��̼��BΪ������CΪ������DΪ������EΪ������̼��FΪˮ��WΪ̼����泥���42���ӵ����ӻ�����������⣮�������ʵ��ܽ�ȿ�֪�ڱ����Ȼ�����Һ��ͨ�������̼�Ͱ���ֻ��������̼�����ƣ���Ϊ̼����������Щ�������ܽ����С��
��1�������ƶϽ����֪EΪ������̼���ṹʽΪ��O�TC�TO��DΪ�����ṹΪ�����ṹ����ʽΪ��

��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3ΪNH3�����ӿռ�ṹΪ�������ͣ�
�ʴ�Ϊ��O�TC�TO��

�������ͣ�
��2��C+D��GΪ�����Ͱ����ϳɰ��ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��N2+3H2
2NH3 ���ʴ�Ϊ��N2+3H2
2NH3
��3��������Ԫ��X��B�е���Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��ҺΪŨ���ᣬ��A����̼��Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��
CO2��+2SO2��+2H2O��
��4��20��ʱ��GΪ������EΪ������̼ͨ�뱥��ʳ��ˮ�У��ǹ�ҵ�Ϻ����Ƽ�����ľ�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��
�ʴ�Ϊ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��
��1�������ƶϽ����֪EΪ������̼���ṹʽΪ��O�TC�TO��DΪ�����ṹΪ�����ṹ����ʽΪ��

��G����Ԫ�ص�ԭ�Ӹ�����Ϊ1��3ΪNH3�����ӿռ�ṹΪ�������ͣ�
�ʴ�Ϊ��O�TC�TO��

�������ͣ�
��2��C+D��GΪ�����Ͱ����ϳɰ��ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��N2+3H2
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
��3��������Ԫ��X��B�е���Ԫ��λ��ͬһ���壬��X������������Ӧˮ�����Ũ��ҺΪŨ���ᣬ��A����̼��Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��
| ||
�ʴ�Ϊ��C+2H2SO4��Ũ��
| ||
��4��20��ʱ��GΪ������EΪ������̼ͨ�뱥��ʳ��ˮ�У��ǹ�ҵ�Ϻ����Ƽ�����ľ�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��
�ʴ�Ϊ��CO2+NH3+NaCl+H2O�TNaHCO3��+NH4Cl��

��ϰ��ϵ�д�
�����Ŀ
 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
 A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���
A��B��C��D��Ϊ������Ԫ����ɵķǽ������ʣ�����B��C��D�ڳ�����Ϊ��̬��AΪ���壬WΪ��42�����ӵ����ӻ��������������ͼ��ת����ϵ����Ӧ�����ԣ���