��Ŀ����
��12�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ����������壬���������������ɱ�״��Ϊ11.2L��

��1�� ��ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������������������������
�������ʽ�����������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ������������������
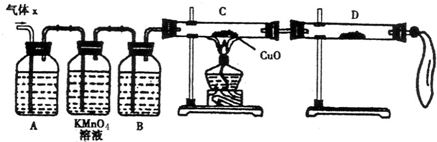
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
��A�м�����Լ�����������������������������������������������������
��B�м�����Լ�������������������
��֤ʵ����X�л����������壬D��Ӧѡ����Լ���������������������ͬʱӦ�۲쵽C�е�ʵ������������������ ��������������������������
��3�����۷�����
�ٸ�С����ͬѧ���ֻ��Ҫ�ٲ��һ�����ݣ�����ȷ��ȷ�����������ɣ�����Ϊ�������Dz����� ��
A����Ӧ��ʣ��п������Ϊ17.5g
B���ռ������������Ϊ25.8g
C��Ũ��������ʵ���Ũ��Ϊ18.0mol/L
�ڸ������ڢ�����ѡ���ݣ�ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ����������������������������������������������
��12�֣�
��1��H2 ��Zn+2H+��Zn2++H2����2�֣�
��2����NaOH��Һ(������������)��������SO2���壬ŨH2SO4
����ˮCuSO4 ����ɫ������
��3����BC����������SO2Ϊ0.4mol��H2Ϊ0.1mol
����������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�