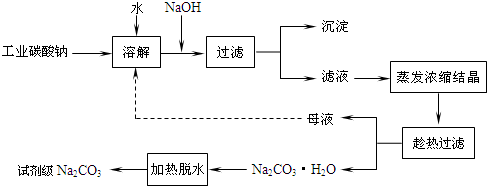
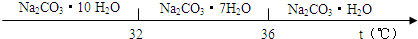��Ŀ����
��̼���Ƶı�����Һ�ڲ�ͬ�¶���������������ͼ��ʾ��

��25��ʱ�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca��OH��2 | Mg��OH��2 | Fe ��OH��3 |
| Ksp | 4.96��10-9 | 6.82��10-6 | 4.68��10-6 | 5.61��10-12 | 2.64��10-39 |
��1������NaOH��Һʱ���������ӷ���ʽΪ______��25��ʱ������Mg2+��Fe3+����Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH=8ʱ��c��Mg2+����c��Fe3+��=______��
��2�������ȹ��ˡ�ʱ���¶�Ӧ������______��
��3�����˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�ã��������ʵ�ʹ�ҵ�������Ƿ����______����˵������______��
��4����֪��Na2CO3?10H2O��s��=Na2CO3��s��+10H2O��g����H=+532.36kJ?mol-1Na2CO3?10H2O��s��=Na2CO3?H2O��s��+9H2O��g����H=+473.63kJ?mol-1д��Na2CO3?H2O��ˮ��Ӧ���Ȼ�ѧ����ʽ______��
| Ksp |
| [OH-]2 |
| 5.61��10-12 |
| 10-12 |
| Ksp |
| [OH-]3 |
| 2.64��10-39 |
| 10-18 |
���� c��Mg2+����c��Fe3+��=5.61��2��.64��10-21=2.215��1021��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����MgCO3+2OH-=Mg��OH ��2��+CO32-�� 2.215��1021��
��2�������ȹ��ˡ���ԭ����ʹ�����ľ���ΪNa2CO3?H2O����ֹ���¶ȹ��Ͷ�����Na2CO3?10H20�����Na2CO3?7H20���壬�����¶ȸ���36�棬�ʴ�Ϊ������36�棻
��3������ĸҺ��ѭ��ʹ�ã�����Һc��Cl-����c��SO42-������������ò���Na2CO3�л������ʣ����������ϸ��ᴿ���գ��ʴ�Ϊ�������У�����ĸҺ��ѭ��ʹ�ã�����Һc��Cl-����c��SO42-������������ò���Na2CO3�л������ʣ�
��4��ͨ���۲������Ȼ�ѧ����ʽ�����ø�˹���ɣ��ɽ���ʽ����õ�Na2CO3?H2O��S���TNa2CO3��s��+H2O��g����
��Na2CO3?H2O��S���TNa2CO3��s��+H2O��g����H=+58.73kJ/mol��
�ʴ�Ϊ��Na2CO3?H2O��S���TNa2CO3��s��+H2O��g����H=+58.73kJ/mol��

 �Ķ��쳵ϵ�д�
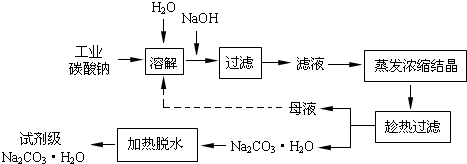
�Ķ��쳵ϵ�д���ҵ̼���ƣ�����ԼΪ98�����к���Ca2+��Mg2+��Fe3+��Cl����SO42�������ʣ��ᴿ������·��ͼ��ʾ��

��̼���Ƶı�����Һ�ڲ�ͬ�¶���������������ͼ��ʾ��

���й����ʵ��ܶȻ�����
|
���� |
CaCO3 |
MgCO3 |
Ca��0H��2 |
Mg��OH��2 |
Fe��OH��3 |
|
Ksp |
4.96��10��9 |
6.82��10��6 |
4.68��10��6 |
5.61��10��12 |
2.64��10��39 |
�ش��������⣺
��1������NaOH��Һʱ����Ӧ�����ӷ���ʽΪ ������Mg2+��Fe3+����Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH=8ʱ��c��Mg2+����c��Fe3+��= ��
��2����ĸҺ���г��˺���Na+��CO32���⣬������ �����ӡ�
��3�����˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ�������������߽���ѭ��ʹ�á����������ʵ�ʹ�ҵ�������Ƿ���У� ������С������С�������˵�����ɣ� ��
��4����֪��Na2CO3��10H2O��s��=Na2CO3��s��+10H2O��g�� =+532.36 kJ��mol��1
=+532.36 kJ��mol��1
Na2CO3��10H2O��s��=Na2CO3��H2O��s��+9H2O��g��  =+473.63
kJ��mol��1
=+473.63
kJ��mol��1
д��Na2CO3��H2O��ˮ��Ӧ���Ȼ�ѧ����ʽ�� ��