��Ŀ����
ij���е�λ���õ绯ѧԭ����SO2���Ʊ����ᣬװ������ͼ������ij�ִ����� �缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���
�缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���
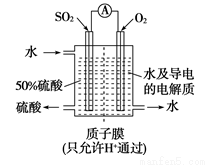
��1��ͨ��SO2�ĵ缫Ϊ__________������缫��ӦʽΪ_________________���˵缫��pH_______(�������С�����䡱����
��2����ͨ��SO2������Ϊ2.24 L/min����״������Ϊ�ȶ����������������� Һ��Ũ��Ӧά�ֲ��䣬�����ˮ������������ ������� mL/min��ʾ��
Һ��Ũ��Ӧά�ֲ��䣬�����ˮ������������ ������� mL/min��ʾ��
��3���Դ˵�Դ�����������������Һ�����������������Ϊ21.6 g�����������������״�������Ϊ__________L��
��4������ʱ��BaSO4��Ksp=1.08��10-10���ֽ��������BaCl2��Һ��3.5��10-3mol/L��Na2SO4��Һ��ϡ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ________��
��5����֪��Fe2O3(s����3C(ʯī��==2Fe(s����3CO(g�� ��H =+489.0 kJ��mol-1
CO(g����1/2O2(g��==CO2(g�� ��H=��283.0 kJ��mol-1
C(ʯī����O2(g��==CO2 (g�� ��H=��393.5 kJ��mol-1
��4Fe(s����3O2(g��==2Fe2O3(s���Ħ�HΪ___________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ʵ������ж��쳣�����ԭ�������û�����ݵ��ǣ�
ѡ�� | �쳣��� | ���ܵ�ԭ�� |
A | �����ᾧʱ������������ | ����Һ���ɣ���ƾ��Ƶ�о����������ײ� |
B | ��Һʱ����Һ©���е�Һ����ѵ��� | û�д�Һ©�����ϵIJ����������ϵİ�����©����С��û�ж��� |
C | ��ȡʱ������Һ�����ò��ֲ� | ��ȡ���ӵ�̫�� |
D | ����ʱ���������������� | û��ͨ����ˮ���ȼ��Ⱥ�ͨ����ˮ |


 ����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5
����ȫȼ�գ��������������Ĺ������ƹ�����ȫ��Ӧ����Ӧ����������ǡ��Ҳ������ng�������������������������������A��H2��CO�Ļ����B��C2H2O2C��C3H6O3D��C6H12O5 ����:
����: �ܱ�������ʢ��������A��B�Ļ�����壬��һ�������·�����Ӧ��A+3B
�ܱ�������ʢ��������A��B�Ļ�����壬��һ�������·�����Ӧ��A+3B 2C����ά���¶Ⱥ�ѹǿ���䣬���ﵽƽ��ʱ�������ݻ�ΪVL������C��������ռ10% �������ƶ���ȷ����
2C����ά���¶Ⱥ�ѹǿ���䣬���ﵽƽ��ʱ�������ݻ�ΪVL������C��������ռ10% �������ƶ���ȷ����
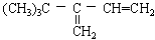
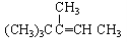
 1��FeCl3����Һ��10mL0.1mol��L��1��KSCN��Һ
1��FeCl3����Һ��10mL0.1mol��L��1��KSCN��Һ