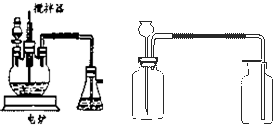
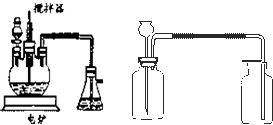
��Ŀ����
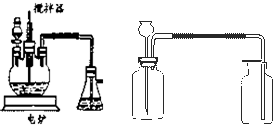
��ҵ����������豸��Ϊ���֣�һ�Ƿ���¯�����ǽӴ��ҡ��������������ڷ���¯�����ջ��������ɶ��������ڽӴ������д��������¶��������һ����������ϣ�������������������������������ʱ������98.3% ��Ũ�������գ�ʹ��������������ˮ�����γ����ᡣ�����װ���Ƿ��չ�ҵ���Ʊ�����Ĺ���������Ƴ����ģ�����̽����ҵ��Ϊ�β���98.3%��Ũ����������������
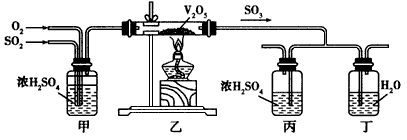
��ش��������⣺
(1)д������¯�����ջ�����ķ�Ӧ����ʽ______________________��
(2)��ͼ�е��ҡ����ֱ��൱�ڹ�ҵ����ȡ����װ���е�________________��_______________��
(3)���е�����Ϊ____________�����е�����Ϊ____________��
(4)��ͼ��ѹǿ��SO2ƽ��ת���ʵ�Ӱ��
(1)д������¯�����ջ�����ķ�Ӧ����ʽ______________________��
(2)��ͼ�е��ҡ����ֱ��൱�ڹ�ҵ����ȡ����װ���е�________________��_______________��
(3)���е�����Ϊ____________�����е�����Ϊ____________��
(4)��ͼ��ѹǿ��SO2ƽ��ת���ʵ�Ӱ��
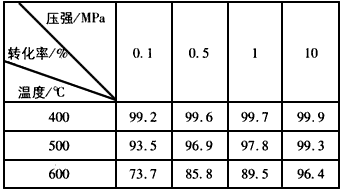
��SO2ת��ΪSO3�ķ�Ӧ������ѹǿ��ʹת����____________��֮����ͨ�����ó�ѹ��������Ϊ____________________________��
(1)4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2
(2)�Ӵ��ң�������
(3)��������������
(4)����ѹ��SO2��ת�����Ѻܴ�
 2Fe2O3+8SO2
2Fe2O3+8SO2 (2)�Ӵ��ң�������
(3)��������������
(4)����ѹ��SO2��ת�����Ѻܴ�

��ϰ��ϵ�д�
�����Ŀ
 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�