��Ŀ����
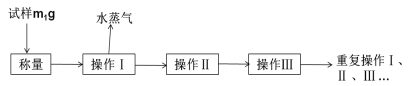
����Ŀ��������ʵ�����вⶨ����ͭ���壨CuSO4��xH2O���ᾧˮ������ʵ�����̡�
�ش���������
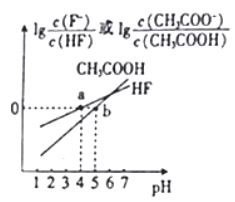
��1��д���������ƣ�������____________������____________
��2���ظ����������_________������Ŀ����_________________
��3��ʵ���������±���

�Լ��㣺����ʵ���ƽ��ֵ x=_______(������С����� 2 λ) ����ʵ�����=______�������
��4����ɱ���ʵ�������ܵ�ԭ����_______��
A������ʱ��������������з�������
B��������ȴʱδ���ڸ�������
C������ͭ�����л������Ȳ��ֽ������
D�����ȹ����У��о��彦��
E��ȡ�õ���Ʒ���ѻ���ǰ��ͬѧ���������ˮ����ͭ
���𰸡���ȴ�� ��������ȷ������ͭ�����нᾧˮȫ��ʧȥ5.214.2%AD
��������
���⿼����ʵ���Ҳⶨ����ͭ����ᾧˮ�ĺ���ʵ�飬��ʧȥˮ�õ�����ķ����ж��Լ���ʵ������������ԭ����з����ǽ��Ĺؼ���
(1)�ⶨ����ͭ�����нᾧˮ�ĺ�����ʵ������������ȡ���ȴ�����������أ����Բ�����_Ϊ��ȴ��������Ϊ������(2) �ظ�������к��أ���Ϊ��ȷ������ͭ�����нᾧˮȫ��ʧȥ��(3)��һ��ʵ��������ͭ������Ϊ37.106-35.688=1.418g��������ͭ�����ʵ���Ϊ1.418/160=0.0088625mol��ˮ������Ϊ37.918-37.106=0.812g��ˮ�����ʵ���Ϊ0.812/18=0.045mol����x=0.045/0.0088625=5.09���ڶ���ʵ���У�����ͭ������Ϊ1.285g�����ʵ���Ϊ1.285/160=0.00803mol��ˮ������Ϊ2.056-1.285=0.771g�����ʵ���Ϊ0.771/18=0.04283mol����x=0.04283/0.00803=5.33����x��ƽ��ֵΪ��5.09+5.33��/2=5.21������ͭ�����к�5���ᾧˮ������������Ϊ��5.21-5��/5=4.2%�� (7) A������ʱ��������������з���������ɼ���ˮ�������ӣ�����ȷ��B��������ȴʱδ���ڸ������У��ⶨˮ�������٣��ʴ���C������ͭ�����л������Ȳ��ֽ�����ʣ���ⶨˮ�������٣��ʴ���D�����ȹ����У��о��彦������Ϊ���ٵĶ�Ϊˮ�����������������ӣ�����ȷ��E��ȡ�õ���Ʒ���ѻ���ǰ��ͬѧ���������ˮ����ͭ����ⶨˮ�������٣��ʴ���ѡAD��

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�