��Ŀ����
(9��)����ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ����������ʱ����һ����̼�����ȼ��ʱ���ɶ�����̼����Ӧ�зų���������ͼ��ʾ��

�ŵ������ʯ��ʯī��ȫȼ��__________������ʯ����ʯī�����ų��������࣬д����ʾʯīȼ���ȵ��Ȼ�ѧ����ʽ ______________________________ ��
����ͨ��״���£����ʯ��ʯī���________������ʯ����ʯī�������ȶ���д��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ __________________________ ��
��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų������� ��
��ż���Һ��������Ҫ�ɷ�֮һ�Ƕ��顣��1g������ȫȼ�ղ�����CO2��Һ̬ˮʱ���ų�����50KJ����д������ȼ�շ�Ӧ���Ȼ�ѧ����ʽ ��
����֪��KOH 28.0gϡ��Һ������ϡ���ᷴӦ���ų�28.65KJ����������д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ ��

�ŵ������ʯ��ʯī��ȫȼ��__________������ʯ����ʯī�����ų��������࣬д����ʾʯīȼ���ȵ��Ȼ�ѧ����ʽ ______________________________ ��
����ͨ��״���£����ʯ��ʯī���________������ʯ����ʯī�������ȶ���д��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ __________________________ ��
��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų������� ��
��ż���Һ��������Ҫ�ɷ�֮һ�Ƕ��顣��1g������ȫȼ�ղ�����CO2��Һ̬ˮʱ���ų�����50KJ����д������ȼ�շ�Ӧ���Ȼ�ѧ����ʽ ��
����֪��KOH 28.0gϡ��Һ������ϡ���ᷴӦ���ų�28.65KJ����������д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ ��
��һ����1�����ʯ��C��ʯī��s����O2(g)��CO2(g) ��H����393.5kJ/mol
��2��ʯī��C��ʯī��s����C�����ʯ��s�� ��H����9.1kJ/mol ��3��252.0kJ
��������1��C4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l) ��H����2900kJ/mol
��2��1/2H2SO4(aq)��KOH(aq)��1/2K2SO4(aq)��H2O(l) ��H����57.3kJ/mol
��2��ʯī��C��ʯī��s����C�����ʯ��s�� ��H����9.1kJ/mol ��3��252.0kJ
��������1��C4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l) ��H����2900kJ/mol
��2��1/2H2SO4(aq)��KOH(aq)��1/2K2SO4(aq)��H2O(l) ��H����57.3kJ/mol
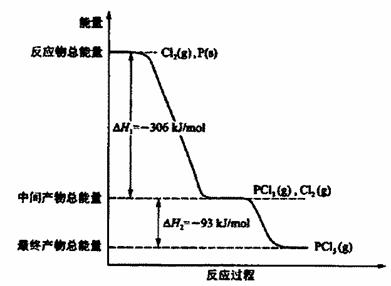
�����������һ����1������ͼ���֪�����ʯ������������ʯī�������������Ե������ʯ��ʯī��ȫȼ�ս��ʯ�ų��������ࡣ����ͼ���֪��1molʯī��ȫȼ�շų���������110.5kJ��283.0kJ��393.5kJ������ʯīȼ���ȵ��Ȼ�ѧ����ʽ��C��ʯī��s����O2(g)��CO2(g) ��H����393.5kJ/mol��
��2�����ʯ������������ʯī��������������ʯī�Ƚ��ʯ�ȶ�������ͼ���֪��1mol���ʯת��Ϊʯī�ų���������395.4kJ��393.5kJ��9.1kJ������ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ��C��ʯī��s����C�����ʯ��s�� ��H����9.1kJ/mol��
��3�������ɵ�CO��CO2���ʵ����ֱ��Ǻ�x��y����x��y��1mol��28x��44y��36g�����x����y��0.5mol������ʵ�ʷų��������ǣ�393.5kJ/mol��110.5kJ/mol����0.5mol��252.0kJ��
��������1����1g������ȫȼ�ղ�����CO2��Һ̬ˮʱ���ų�����50KJ����1mol���鼴58g������ȫȼ�շų���������50KJ��58��2900kJ�����Է�Ӧ���Ȼ�ѧ����ʽ��C4H10(g)��13/2O2(g)��4CO2(g)��5H2O(l) ��H����2900kJ/mol��
��2���к�������һ�������£�ϡ��Һ�У�ǿ���ǿ�Ӧ����1molˮʱ���ų���������28g�������ص����ʵ�����0.5mol�����Ը÷�Ӧ���Ȼ�ѧ����ʽ��1/2H2SO4(aq)��KOH(aq)��1/2K2SO4(aq)��H2O(l) ��H����57.3kJ/mol��
�����������Ǹ߿��еij������㣬�����е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ�����ѵ��������������ѧ����������������Ӧ�����������ѧ����ѧϰЧ�ʡ�����Ĺؼ����˽ⷴӦ�ȵĺ��壬Ȼ����ͼ��������á����������㼴�ɡ�

��ϰ��ϵ�д�
�����Ŀ
 cC��g��+ dD��g����������ͼʾ�ش�
cC��g��+ dD��g����������ͼʾ�ش�