ƒøƒ⁄»›
ø…ƒÊ∑¥”¶2NH3 N2+3H2‘⁄√‹±’»›∆˜÷–Ω¯––£¨¥ÔµΩ∆Ω∫‚◊¥Ã¨µƒ±Í÷æ «£® £©
N2+3H2‘⁄√‹±’»›∆˜÷–Ω¯––£¨¥ÔµΩ∆Ω∫‚◊¥Ã¨µƒ±Í÷æ «£® £©
¢Ÿµ•Œª ±º‰ƒ⁄…˙≥…n mol N2µƒÕ¨ ±…˙≥…2n mol NH3 ¢⁄µ•Œª ±º‰ƒ⁄…˙≥…n mol N2µƒÕ¨ ±…˙≥…3n mol H2 ¢€”√NH3°¢N2°¢H2µƒŒÔ÷ µƒ¡ø≈®∂»±‰ªØ±Ì 浃∑¥”¶ÀŸ¬ ÷Ʊ»Œ™2°√1°√3 ¢‹∏˜∆¯Ãµƒ≈®∂»≤ª‘Ÿ∏ƒ±‰ ¢›ªÏ∫œ∆¯Ãµƒ∆Ωæ˘œ‡∂‘∑÷◊”÷ ¡ø≤ª‘Ÿ∏ƒ±‰?
| A£Æ¢Ÿ¢‹¢› | B£Æ¢⁄¢€¢›? | C£Æ¢Ÿ¢€¢‹ | D£Æ¢Ÿ¢⁄¢€¢‹¢›? |
A
Ω‚Œˆ

¡∑œ∞≤·œµ¡–¥∞∏
œ‡πÿƒø
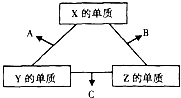 X°¢Y°¢Z»˝÷÷∂Ã÷‹∆⁄‘™Àÿ£¨À¸√«µƒ‘≠◊”–Ú ˝÷Æ∫ÕŒ™16£Æ‘⁄≥£Œ¬œ¬£¨X°¢Y°¢Z»˝÷÷‘™Àÿµƒ≥£º˚µ•÷ ∂º «Œfi…´∆¯Ã£¨À¸√«‘⁄ µ±Ãıº˛œ¬ø…∑¢…˙»ÁÕºÀ˘ 浃◊™ªØ£Æ“—÷™“ª∏ˆB∑÷◊”÷–∫¨”–Z‘™Àÿµƒ‘≠◊”∏ˆ ˝±»“ª∏ˆC∑÷◊”÷–∫¨”–Z‘™Àÿµƒ‘≠◊”∏ˆ ˝…Ÿ“ª∏ˆ£Æ«Îªÿ¥œ¬¡–Œ £∫
X°¢Y°¢Z»˝÷÷∂Ã÷‹∆⁄‘™Àÿ£¨À¸√«µƒ‘≠◊”–Ú ˝÷Æ∫ÕŒ™16£Æ‘⁄≥£Œ¬œ¬£¨X°¢Y°¢Z»˝÷÷‘™Àÿµƒ≥£º˚µ•÷ ∂º «Œfi…´∆¯Ã£¨À¸√«‘⁄ µ±Ãıº˛œ¬ø…∑¢…˙»ÁÕºÀ˘ 浃◊™ªØ£Æ“—÷™“ª∏ˆB∑÷◊”÷–∫¨”–Z‘™Àÿµƒ‘≠◊”∏ˆ ˝±»“ª∏ˆC∑÷◊”÷–∫¨”–Z‘™Àÿµƒ‘≠◊”∏ˆ ˝…Ÿ“ª∏ˆ£Æ«Îªÿ¥œ¬¡–Œ £∫ £®2009?«Â‘∂ƒ£ƒ‚£©µ¬π˙»Àπ˛≤Æ‘⁄1909ƒÍ∑¢√˜µƒ∫œ≥…∞±∑¥”¶‘≠¿ÌŒ™£∫N2£®g£©+3H2£®g£©?2NH3£®g£© “—÷™298K ±£∫°˜H=-92.4kJ?mol-1 ‘ªÿ¥œ¬¡–Œ £∫
£®2009?«Â‘∂ƒ£ƒ‚£©µ¬π˙»Àπ˛≤Æ‘⁄1909ƒÍ∑¢√˜µƒ∫œ≥…∞±∑¥”¶‘≠¿ÌŒ™£∫N2£®g£©+3H2£®g£©?2NH3£®g£© “—÷™298K ±£∫°˜H=-92.4kJ?mol-1 ‘ªÿ¥œ¬¡–Œ £∫