��Ŀ����
ɽ������һ�ֳ��õ�ʳƷ��������������ɽ�����һ�ֹ�ҵ�ϳ�;����
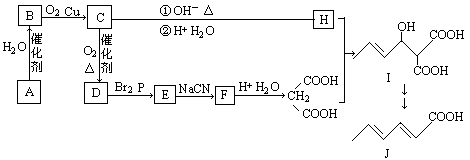 ��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O
��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O
��2��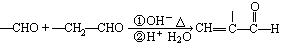
��3��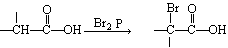
��4��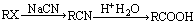
�ش��������⣺
��1��E�Ľṹ��ʽΪ �����й����ŵ����� ��
��2��G��H��һ�������ºϳ�I�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
��3��д��������������ɽ���������ͬ���칹��
�ٷ���������Ԫ̼�����ں˴Ź����������ĸ��壻�۷������С�COO���ṹ
��4������˵����ȷ���� ��
A��I���Է����ӳɡ�ȡ������������ȥ��Ӧ B��I���Ժ����Ƶ�������ͭ��Ӧ
C��1molI��ȫȼ�տ�������7molO2 D��J�͵����ʵ�����H2�ӳ������ֲ���
|
 ��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O
��֪����1��A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ����C�ķ���ʽΪC2H4O��2��
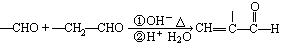
��3��
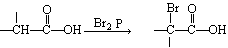
��4��
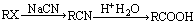
�ش��������⣺
��1��E�Ľṹ��ʽΪ �����й����ŵ����� ��
��2��G��H��һ�������ºϳ�I�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
��3��д��������������ɽ���������ͬ���칹��
�ٷ���������Ԫ̼�����ں˴Ź����������ĸ��壻�۷������С�COO���ṹ
��4������˵����ȷ���� ��
A��I���Է����ӳɡ�ȡ������������ȥ��Ӧ B��I���Ժ����Ƶ�������ͭ��Ӧ
C��1molI��ȫȼ�տ�������7molO2 D��J�͵����ʵ�����H2�ӳ������ֲ���
��1��BrCH2COOH ��ԭ�� �Ȼ�
��2��CH3CH=CHCHO+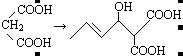 �ӳɷ�Ӧ
�ӳɷ�Ӧ
��3��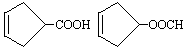
��4��ABC
��2��CH3CH=CHCHO+
 �ӳɷ�Ӧ
�ӳɷ�Ӧ��3��
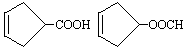
��4��ABC
���������������Ŀ������Ϣ���л��ϳ�·�߽����Ƶ���A�Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ������AΪCH2=CH2��CH2=CH2��H2O��Ӧ����B����BΪCH3CH2OH��CH3CH2OH��O2��Cu������������������C����CΪCH3CHO��HΪCH3CH=CHCHO��DΪCH3COOH��EΪBrCH2COOH��FΪNCCH2COOH��
��1��E�Ľṹ��ʽΪ��BrCH2COOH�����й����ŵ�����Ϊ����ԭ�Ӻ��Ȼ���
��2�������л��ϳɿ�ͼ��G��H������ȩ���Ϸ�Ӧ����I����ѧ����ʽΪ��
CH3CH="CHCHO+"
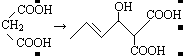 ��Ӧ����Ϊ�ӳɷ�Ӧ��
��Ӧ����Ϊ�ӳɷ�Ӧ����3�����ݢٷ���������Ԫ̼�����ں˴Ź����������ĸ��壬˵����������λ�õ�Hԭ�ӣ��۷������С�COO���ṹ���ɵ�����ͬ���칹�塣
��4��I����̼̼˫�����ǻ����Ȼ������Կɷ����ӳɡ�ȡ������������ȥ��Ӧ����ȷ��B��I�����Ȼ������������Ƶ�Cu(OH)2�����к�Ӧ����ȷ��C��I�ķ���ʽΪC7H10O5������1mol I��ȫȼ�տ�������7molO2����ȷ��D��J�����ں���2��̼̼˫�����͵����ʵ�����H2�ӳ������ֲ������

��ϰ��ϵ�д�
 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�
�����Ŀ

 ��5��F��G�ķ�Ӧ������ ��
��5��F��G�ķ�Ӧ������ ��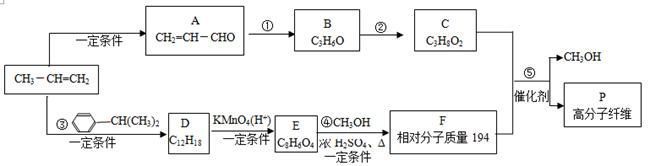
 RCO18OR"��R'OH
RCO18OR"��R'OH
 �Ľṹ���Ա�ʾΪ
�Ľṹ���Ա�ʾΪ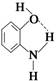 (���߱�ʾ���)�����л���
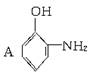
(���߱�ʾ���)�����л���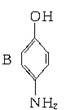 ֻ���γɷ��Ӽ��������ҵ����ˮ��������A��B���з��룬�����ȱ��������ijɷ���__________��
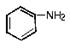
ֻ���γɷ��Ӽ��������ҵ����ˮ��������A��B���з��룬�����ȱ��������ijɷ���__________��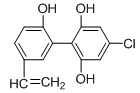
 ������Ⱦ��ķ�ˮ�����ɻ��������վ�����˹�뺣��
������Ⱦ��ķ�ˮ�����ɻ��������վ�����˹�뺣��