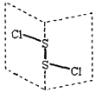
��Ŀ����
����Ŀ��ǰ������Ԫ��A��B��C��D��E��Fԭ���������ε�������֪��A��B��D��C�ļ۵������������࣬A��B��Cͬ������C�Ǹ������е縺������Ԫ�أ�A�����������ӣ�E��δ�ɶԵ�������ǰ�����������ģ�����۵�������D��ͬ�� F������������Ϊ2���ڲ�ȫ�����������ö�Ӧ��Ԫ�ط��Żش���������⣺
��1��д��E�ļ۵����Ų�ʽ��______________��
��2����A�γɵĻ������У�A��ȡsp2�ӻ����ҷ�������С�Ļ�����Ϊ��д��ѧʽ��______________��
��3���������ʵ�����������йص���______________��
A. ��ȼ�����γ� B. A���⻯��ķе� C. B���⻯������ȶ���
��4��E3+������AB���γ������ӣ�����E3+��d2sp3��ʽ�ӻ����ӻ����ȫ��������AB���γ���λ������E3+����λ��Ϊ______________��1mol���������к���______________mol������
��5��F��D�γɵĻ����ᄃ����ͼ��F����λ��Ϊ______________�������ܶ�Ϊa g/cm3,NAΪ�����ӵ����������߳�Ϊ______________pm����1pm=10-10cm��

���𰸡�3d54s1 C2H4 A 6 12 4 ![]()
��������
���Ȿ�⿼�����ʵĽṹ�����ʡ��漰Ԫ�ص��ƶϣ��۵����Ų�ʽ����д���������λ����ȷ���ͦҼ��ļ��㣬�����ķ����ͼ��㡣E��δ�ɶԵ�������ǰ�����������ģ�EΪCrԪ����F��ԭ����������E��F������������Ϊ2���ڲ�ȫ��������F��̬ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s2��FΪZnԪ����E�ļ۵�����Ϊ6��D�ļ۵�������E��ͬ��D�ļ۵�����Ϊ6��A��B��Cͬ���ڣ�A��B��C��Dԭ���������ε�����A��B��D��C�ļ۵���������������A��B��CΪ�ڶ����ڣ�DΪ�������ڣ�DΪSԪ�أ�A��B��CΪ�ڶ�������C�Ǹ������е縺������Ԫ����CΪFԪ�أ�A�ļ۵�����С��B��B�ļ۵�����С��6��A�����������ӣ���AΪCԪ�أ�BΪNԪ�ء�
��1��EΪCr��Cr��ԭ������Ϊ24������ԭ�Ӻ�������Ų����ɣ�E�ĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1���۵����Ų�ʽΪ3d54s1��
��2��AΪCԪ�أ���A�γɵĻ�������A��ȡsp2�ӻ�����������С�Ļ�����ΪCH2=CH2����ѧʽΪC2H4��
��3��A����ȼ��������Ȼ����ˮ�ڸ��µ�ѹ�������γɵ����״�����ʣ���ȼ����ˮ���Ӽ�ͨ������γ���״�ṹ����������CH4���ӻ�H2O���ӣ���ȼ�����γ�������йأ�B��AΪCԪ�أ�C�ĵ縺�Խ�С��A���⻯����Ӽ䲻�γ��������е�ĸߵ�������أ��뷶�»����йأ�C��BΪNԪ�أ�B���⻯������ȶ����빲�ۼ��ļ����йأ�����Ӽ�����أ�������йص���A����ѡA��
��4��EΪCr��Cr3+��d2sp3��ʽ�ӻ����γ�6���ӻ�������ӻ����ȫ��������AB-��CN-���γ���λ����Cr3+����λ��Ϊ6��6����λ��ȫΪ�Ҽ�������CN-�ĵ���ʽΪ![]() ��1������CN-��1���Ҽ���2���м�����1mol��������[Cr��CN��6]3-�к��еĦҼ�Ϊ��6+6
��1������CN-��1���Ҽ���2���м�����1mol��������[Cr��CN��6]3-�к��еĦҼ�Ϊ��6+6![]() 1��mol=12mol��
1��mol=12mol��
��5��FΪZn��DΪS��������̯������������С����ĸ���Ϊ4������ĸ���Ϊ8![]() +6
+6![]() =4���þ���Ļ�ѧʽΪZnS��Zn��S����λ����ȣ��ɾ�������С�������λ��Ϊ4����F����λ����Ϊ4��1mol���������Ϊ��65+32��g=97g��1mol��������Ϊ
=4���þ���Ļ�ѧʽΪZnS��Zn��S����λ����ȣ��ɾ�������С�������λ��Ϊ4����F����λ����Ϊ4��1mol���������Ϊ��65+32��g=97g��1mol��������Ϊ![]() =
=![]() cm3�����������Ϊ4
cm3�����������Ϊ4![]() ��
��![]() cm3
cm3![]() NA��=
NA��=![]() cm3�������ı߳�Ϊ
cm3�������ı߳�Ϊ![]() cm=
cm=![]() 1010pm��
1010pm��

 С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�