��Ŀ����
��10�֣���������Ҫ�ɷ�ΪFeS2�������ҹ���������᳧��ȡ�������Ҫԭ�ϡ�ij��ѧѧϰС��Ի�����ʯ��������ʵ��̽����
[ʵ��һ]�ⶨ��Ԫ�صĺ�����
I����m1 g�û�������Ʒ������ͼ��ʾװ�ã��гֺͼ���װ��ʡ�ԣ���ʯӢ���У���a�����ϵػ���ͨ���������������ʯӢ���еĻ�������Ʒ����Ӧ��ȫ��ʯӢ���з�����Ӧ�Ļ�ѧ����ʽΪ��4FeS2��11O22Fe2O3��8SO2
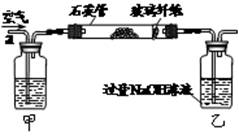
II����Ӧ��������ƿ�е���Һ��������ͼ��ʾ������
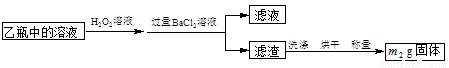
[ʵ���]�ⶨ��Ԫ�صĺ�����
III���ⶨ��Ԫ�صĺ�����ʵ�鲽������ͼ��ʾ��

�������ۣ�
��1������װ���У���ƿ����ʢ�Լ��� ��Һ��
��2������ƿ��Һ�еμ�H2O2��Һʱ������������ԭ��Ӧ�����ӷ���ʽΪ ��
II�г��˿�����H2O2��Һ��Ϊ�������������Լ��� ������ţ���
����ˮ ��ŨH2SO4 ��HNO3 ��Fe2(SO4)3
��3���û���������Ԫ�ص���������Ϊ ��
��4��III�IJ�����У���ѡ����������ԭ��������Ϊ�����𣿲�˵�����ɡ������������˿ղ�� ��������������������� �����������˿ղ����
��5����Ҫ�ⶨ��Ԫ�صĺ���������III�л���Ҫ�ⶨ�������� ��
��1��NaOH����KOH����1�֣�
��2��SO32����H2O2��SO42����H2O��2�֣� �٢ۣ�2�֣�
��3��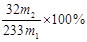 ��2�֣�
��2�֣�
��4����������1�֣������ۻ���Fe2 (SO4)3 ��H2SO4��Ӧ����Fe2����ʹ�������Ԫ�ص���������ƫ��1�֣�
��5����������������KMnO4��Һ�������1�֣�
��������

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| |||||||||||||||||||
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�

�����ϵ�֪��
|
�������� |
�ܶȻ�(Ksp) |
pHֵ |
|
|
��ʼ���� |
��ȫ���� |
||
|
Mg(OH)2 |
5.6��10��12 |
9.3 |
10.8 |
|
Fe(OH)3 |
2.8��10��16 |
2.7 |
3.7 |
|
Al(OH)3 |
1.3��10��33 |
3.7 |
4.7 |
��ش��������⣺
��1��д������A�Ļ�ѧʽΪ ��
��2����Ҫ�ⶨ��Һ��pH�Ƿ�ﵽ3.7������ʵ����Ʒ�п�ѡ�õ��� ��
A��ʯ����Һ B���㷺pH��ֽ C������pH��ֽ D��pH��
��3������������ӷ�Ӧ����ʽ
����ҺD���ɹ���E �� ����ҺF���ɹ���G ��
��4��Ҫ������C������E����G��ת��Ϊ��Ӧ���ȶ����������е�ʵ�����Ϊ ��
��5������������Һ����ı仯���������ҺH��c��Mg2����= ��